Common Questions When Developing an Environmental Monitoring Program for a Food Facility
ID
FST-445NP
What is an Environmental Monitoring Program?
An environmental monitoring program (EMP) is a written document that includes pathogen, indicator organisms, or index organisms monitoring and testing to detect microbial risks in the food processing environment. The presence of indicator organisms provides information related to the general hygienic quality of the environment (Chapin, 2014; Kornacki, 2011). On the other hand, index organisms whose presence relates to the possible occurrence of pathogens that can survive under similar nutrient, chemical, and physical conditions (Chapin, 2014; Kornacki, 2011). Environmental monitoring is an essential tool for understanding how production processes and hygiene status impact food safety risks due to environmental hazards in your food facility. The goal of this document is to answer common questions regarding EMPs and to help producers understand how to begin developing and implementing EMPs in their facilities.
Why Should My Facility Have An EMP?
Under the Preventive Controls for Human Food regulation (PCHF Rule), facilities that produce ready-to-eat (RTE) products that identify an environmental hazard requiring a preventive control are required to use environmental monitoring as a verification activity (FDA, 2020). An RTE food is a food that will not be cooked or further processed in a way that will reduce the load of organisms of concern; some examples include salads, cooked meats, smoked fish, cheese, spices, and produce. Since ready-to-eat foods are typically not cooked by the consumer before consumption, it is important to decrease the risk of contamination by harmful pathogens in a facility. EMPs can be used as a verification tool for cleaning and sanitizing activities as a means of controlling environmental hazards. Before an EMP is put in place, it is important to ensure that you have a comprehensive sanitation program. Your environmental monitoring program is a verification of the effectiveness of your cleaning and sanitizing. A comprehensive sanitation program includes validation of cleaning and sanitizing activities, employee training, written SOPs, and appropriate recordkeeping practices.
What Should My EMP Test For?
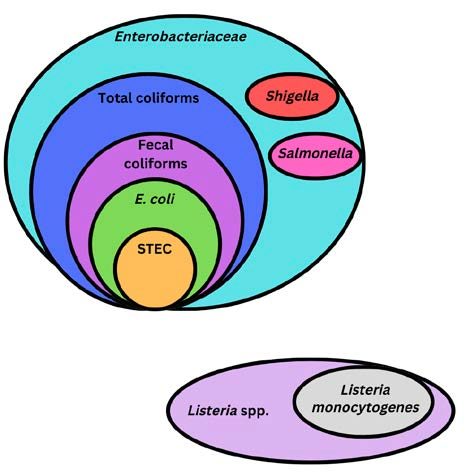
What your EMP should test for depends on the goals of your EMP. Typical microbial targets for EMPs include ATP, spoilage organisms, indicator organisms, and/or pathogens. Additionally, EMPs can be for specific allergens. Table 1 outlines target organisms and the goal of each target.
Target |
Purpose |
|---|---|
ATP |
ATP testing methods are a simple and rapid way to determine the hygienic status of surfaces in your processing facility. |
Spoilage organisms |
Spoilage organism testing can be used to verify sanitation methods in the processing environment. Examples of spoilage organisms include yeasts and molds. |
Indicator organisms |
Indicator organism testing can be used to determine the hygienic status of your facility, understand the microbial ecology of the processing environment, verify sanitation, and assess post- processing contamination risk. Examples of indicator organisms include total coliforms, fecal coliforms, and Enterococci. |
Foodborne pathogens |
Pathogen testing is used to identify and eliminate environmental pathogen sources. Examples of foodborne pathogens include Salmonella and Listeria monocytogenes |
Allergens |
Allergen testing can be used to confirm that there is no allergen residue on your equipment and/or in your facility to prevent allergen cross-contamination. This would only be a consideration if you were processing both allergen-containing and non-allergen-containing products on the same equipment. |
What Components Should My EMP Consist Of?
While An EMP must be designed and tailored specifically for the facility in which it is going to be used, all EMPs should outline the EMP team, contain a map of the facility, detail the process flow, outline key parameters, establish corrective action plans, and define recording-keeping requirements.
Table 2 outlines each of these components.
Component |
Comments |
|---|---|
EMP Team |
Create a cross-functional team that includes people within the business that have expertise in areas such as food safety, quality assurance, maintenance, production, etc. |
Facility Map |
A map and floor plans of your facility separated into hygienic zones |
Process Flow |
All the steps that raw materials go through to become a finished product, including details on steps and equipment |
Key Parameters |
Key parameters, including target organisms, testing procedures, sample sites, sampling frequency, number of samples collected per sampling time, and testing lab |
| Corrective Action Procedures | A corrective action is an action to eliminate the root cause of an undesirable situation to prevent recurrence. In your EMP, this is addressing how you will respond if environmental monitoring identifies that you are outside of a limit for a target |
| Records | Records including training records, SOPs, results of testing, and corrective actions |
Where Should I/We Sample in My Facility?
Sampling sites should be selected with the goal of finding potential issues rather than selecting sites that are easy to clean/sanitize and will always meet acceptable limits. Since the objective of an EMP is to detect potential sources of contamination, sampling typically focuses on areas of greatest concern. This allows you to have a better chance of finding potential problems in the processing environment.
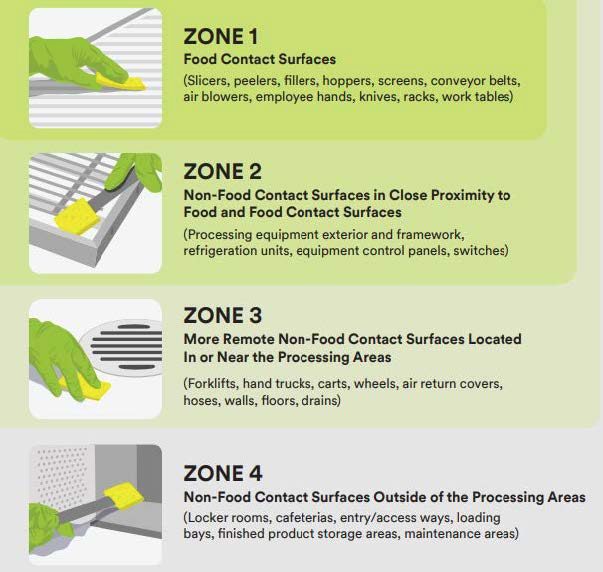
All EMPs use the concept of sampling “zones”, with locations within a facility assigned to one of four zones. Zone 1 represents surfaces that directly contact food while Zone 4 represents areas outside of the processing environment (Figure 2). Best practices focus on monitoring sampling sites for a mixture of zones 2-4.
When you develop your EMP, you should make a master list of possible sampling sites. It is important to look at areas that present the greatest risk; thus, this master list should include locations that are hard to clean, high-traffic areas, and pathways that may facilitate pathogen movement in the facility. It is also important that your master list of sampling locations include sites from each processing step. Examples of sampling locations include:
Cracks, crevices, and other hard-to-reach places

Tight corners, bends, and sharp edges
Areas that are exposed to the movement of people, trash, and/or forklifts
Areas that routinely have pooling water or are collection points of debris

Locations after the kill step
Problem areas or areas that have historically had positive or out-of-specification results
While your food processing facility may have a master list of 500 sites, you may only collect samples from 50 randomly selected sites during each sampling event. The facility size, number of lines, and various activities may affect the number of sites on a master list and the number of sites tested each time. It is also important to vary the sites tested, paying less attention to sites that routinely test negative or are within acceptable limits. Lastly, it is encouraged to collect samples that are not included in the master sample site list, including high-risk sites like pooled water or new cracks in the floor that may become apparent.
How Often Should I Collect Samples?![]()
The frequency of sampling in your facility should be based on your risk, taking into consideration how often you clean and sanitize, the product’s intrinsic properties, the level of risk at each process step, the amount of product produced, and your facility history. For example, facilities where RTE foods that support pathogen growth are exposed to the environment would be considered high-risk and would require more frequent sampling (i.e., weekly). There is no “right” answer as to the frequency of sampling.
What Should I Do If Test Result Are Positive or Above Acceptable Limits?
When you get a positive sample or a microbial count above the acceptable limits in your environmental monitoring, you will enact the corrective actions you’ve outlined in your EMP to eliminate the source of contamination. All unacceptable test results should prompt corrective action. Depending on the type of result received, corrective actions may include re-cleaning, re-testing, repairing or replacing equipment, holding product, and/or possibly a product recall. Additionally, it is important to determine the root cause of the contamination, possibly by conducting concentrated sampling in that area and surrounding areas.
References and Resource
3M and Cornell University. Environmental Monitoring Handbook for the Food and Beverage Industries. Available at https://multimedia.3m.com/mws/media/1684575O/environmental-monitoring-handbook.pdf
Chapin, T. K., Nightingale, K. K., Worobo, R. W., Wiedmann, M., & Strawn, L. K. (2014). Geographical and meteorological factors associated with isolation of Listeria species in New York State produce production and natural environments. Journal of Food Protection, 77(11), 1919-1928.
Food and Drug Administration (2020). FSMA Final Rule for Preventive Controls for Human Food. Available at: https://www.fda.gov/food/food-safety-modernization-act-fsma/fsma-final-rule-preventive-controls-human-food
International Fresh Produce Association (formally United Fresh). Guidance on![]()
Environmental Monitoring and Control of Listeria for the Fresh Produce Industry. Available at: https://www.centerforproducesafety.org/amass/d ocuments/document/263/Listeria%20Guidance%20UFPA%202013.pdf
Kornacki, J. L. (2011). Indicator organism assays: chaos, confusion and criteria. Food Saf. Mag, 17, 24-26.
Publication Date
March 9, 2023



