Tick-Borne Diseases in Virginia
ID
ENTO-504NP
Introduction
Ticks are hematophagous (blood-feeding) arthropods and prolific vectors of a variety of pathogens, with tick populations established across the United States, including 20 hard-bodied tick species residing in Virginia. Each tick species is associated with the transmission of different specific pathogens of both medical and veterinary importance.
In Virginia, there are 4 major human-biting tick species: Ixodes scapularis (the blacklegged tick), Dermacentor variabilis (the American dog tick), Amblyomma americanum (the lone star tick), and Amblyomma maculatum (the Gulf Coast tick).
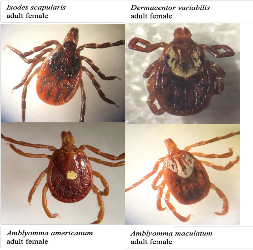
The blacklegged tick is responsible for the transmission of pathogens including Borrelia burgdorferi (causative agent of Lyme disease), Borrelia miyamotoi (agent of tick-borne relapsing fever), Anaplasma phagocytophilum (agent of anaplasmosis), Babesia microti (agent of babesiosis), and Powassan virus. The tick species is active year-round with the nymphal life stage actively seeking hosts in the spring and summer, and the adult life stage active in the winter.
The American dog tick is responsible for the transmission of several rickettsial bacteria, notably including the Rickettsia rickettsii (causative agent of Rocky Mountain spotted fever). Only the adult life- stage of this tick species bite humans, and they are active during the spring and summer.
The lone star tick is responsible for the transmission of pathogens including Ehrlichia chaffeensis and Ehrlichia ewingii (causing ehrlichiosis), Heartland virus, Bourbon virus, an unknown agent involved in Southern Tick-Associated Rash Illness (STARI); as well as alpha-gal - not a pathogen but a sugar in the saliva of this tick species responsible for allergic reactions to red meat. All life stages of this tick species are active in the spring and summer.
The Gulf Coast tick is responsible for transmission of Rickettsia parkeri (causing R. parkeri rickettsiosis; also referred to as Tidewater Spotted Fever).3 Adults of this tick species are active during the summer. The ticks populations vary across the State of Virginia dependent on habitat type (typically secondary successional forests and disturbed grasslands) and animal host presence.
Ehrlichiosis
Ehrlichiosis is a (potentially under-reported1) tick- borne infection caused by the bacteria Ehrlichia chaffeensis or E. ewingii in humans. The main tick species associated with spreading this pathogen is the lone star tick. Infection with Ehrlichia bacteria can cause flu-like symptoms and more severe disease in immuno-compromised individuals. Typical disease reporting for ehrlichiosis occur during the summer months when the lone star tick is most active.
Lyme disease
Borrelia burgdorferi is a spirochete bacterium responsible for causing Lyme disease in the US. Cases of Lyme disease occur year-round with heightened case counts during the summer months when nymphs of the blacklegged tick are active. Once an infected blacklegged tick (adult or nymph) bites a human or animal, the time for B. burgdorferi to move from the tick midgut to its salivary glands and into a new host is estimated as 24-72 hours meaning that the tick would need to be attached for at least that long in order to transmit the bacterium. Cases of Lyme disease have a higher prevalence in northern and south-west Virginia potentially due to some of the following factors: the presence of reservoir hosts such as white-footed mice, the presence of the aggressive phenotype of the blacklegged tick which is more likely to bite humans, and specific habitat and climate conditions influencing this tick’s ability to survive overwinter.
Tick-borne viruses
Powassan, Heartland, and Bourbon viruses are three serious tick-borne pathogens emerging in Virginia which could result in fatality. Please refer to VCE Publication: Emerging Tick-Borne Arboviruses: Powassan virus, Heartland virus, and Bourbon virus (ENTO-491) for further details.
Spotted Fever Group Rickettsiosis (SFGR)
There are two main groups of Rickettsia bacteria: typhus and spotted fever. Typhus group species are typically transmitted by lice and fleas whereas spotted fever group rickettsia are transmitted by ticks. In Virginia, human cases of SFGR are common, with 300-400 cases per year. The causative agents can be transmitted by several tick species including the American dog tick, the lone star tick, and the Gulf Coast tick. Disease symptoms vary depending on the species of infecting bacteria (R. rickettsii and R. parkeri being most common). In a severe SFGR case (e.g., Rocky Mountain spotted fever), immediate treatment is necessary as the infection can move beyond a severe spotted rash and spread to internal organs and past the blood-brain barrier, resulting in death. SFGR from R. parkeri causes milder symptoms and include the presence of eschars on the skin followed by flu-like symptoms.
Southern tick-associated rash illness (STARI)
STARI is a skin condition that can occur after being bitten by the lone star tick. It is unknown what agent is responsible for infection, but the symptoms of STARI often mimic that of the initial stages of Lyme disease including fatigue, fever, muscle and joint pain, headache, and rash.
Alpha-gal Syndrome (Red meat allergy)
The saliva of one tick species (the lone star tick) can cause an immune response in the human body via antibodies produced in allergic reactions. Alpha-gal (galactose-α-1,3-galactose) is a sugar present in the saliva of all life-stages of the lone star tick, and this same sugar is present in many mammalian tissues (including pork, beef, lamb, and venison).
Not everyone who is bitten by the lone star tick will develop an allergic reaction and there are many unknown factors as to why certain people are susceptible to developing alpha-gal syndrome while others are not. Since the alpha-gal sugar is consumed through food instead of exposure through a tick bite, the allergic reaction is delayed as your body digests red meat and dairy products containing the sugar. Alpha-gal syndrome is characterized by extreme stomach pain, hives, nausea and vomiting, diarrhea, swelling of the lips, face, and tongue, and in severe cases can result in anaphylaxis.
Treatment and Tick Prevention
In disease cases where a bacterial pathogen is involved (Lyme disease, Ehrlichiosis, or a SFGR), antibiotics (e.g., Doxycycline) is the preferred method of treatment. Always talk to your doctor if you develop flu-like symptoms after exposure to tick bites. Tick-borne viruses (i.e., Heartland, Powassan, or Bourbon viruses) do not respond to antibiotics.
For all tick-borne illnesses or alpha-gal syndrome, tick prevention is the best method to prevent these illnesses from occurring in the first place or for reoccurrence of symptoms. When going into tick habitat (including fields, forest, and trail edges), dressing appropriately, and applying proper repellents is the best way to prevent tick bite exposures. Wearing long-sleeved and long-pant light colored clothing, tucking your pant legs into your socks, and your shirt into your pants are useful ways to prevent tick access to your bare skin. Also, the treatment of clothing with 0.5% permethrin is a useful method to kill ticks on contact when they get on your clothing. Use only as directed and do not apply permethrin directly on the skin.
References
Centers for Disease Control and Prevention (CDC). Diseases transmitted by ticks. https://www.cdc.gov/ticks/diseases/index.html. Accessed 4 May 2022.
Commins SP and Platts-Mills TA (2013). Delayed anaphylaxis to red meat in patients with IgE specific for galactose alpha-1, 3-galactose (alpha- gal). Current allergy and asthma reports, 13(1), pp.72-77.
Paddock CD, Sumner JW, Comer JA, Zaki SR, Goldsmith CS, Goddard J, McLellan SL, Tamminga CL, Ohl CA (2004) Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clinical Infectious Diseases, 38(6), pp805-811.
Eisen L (2018) Pathogen transmission in relation to duration of attachment by Ixodes scapularis ticks. Ticks and tick-borne diseases, 9(3), pp.535- 542.
Donahue JG, Piesman C, Spielman A (1987) Reservoir competence of white-footed mice for Lyme disease spirochetes. Amer J Trop Med Hyg 36(1) pp92-96.
Arsnoe I, Tsao JI, Hickling GJ (2019) Nymphal Ixodes scapularis questing behavior explains geographic variation in Lyme borreliosis risk in the eastern United States. Ticks and tick-borne diseases, 10(3) pp553-563.
Virginia Department of Health (VDH). Virginia reportable disease surveillance data. Accessed 4 May 2022. https://www.vdh.virginia.gov/surveillance-and-investigation/virginia-reportable-disease- surveillance-data/.
Egizi A, Fefferman NH, Jordan RA (2017) Relative risk for Ehrlichiosis and Lyme disease in an area where vectors for both are sympatric, New Jersey, USA.
Centers for Disease Control and Prevention (CDC). Tick bite prophylaxis. Accessed 4 May 2022. https://www.cdc.gov/ticks/tickbornediseases/tick-bite-prophylaxis.html.
Acknowledgements
The authors acknowledge HATCH project fund VA- 160131, as well the CALS Strategic Plan Integrated Seed Grant 2021 awarded to Gillian Eastwood (VT Dept of Entomology), Alexandra Cumbie (VT Dept of Entomology), Kevin Lahmers (Virginia & Maryland College of Veterinary Medicine), Omar Saucedo (VT Dept of Mathematics), Tom Stanley (VCE Rockbridge County), Tim Mize (VCE Fauquier County), and Leslie Prillaman (VCE Roanoke Unit). We would also like to thank past and present members of the Eastwood Disease Ecology for assistance with tick collections and analysis, in particular Lucas Raymond, Rebecca Trimble, Lindsey Faw, Christian Reid, Amanda Whitlow, Mikayla Hearne, and Peter Schiff.
Virginia Cooperative Extension materials are available for public use, reprint, or citation without further permission, provided the use includes credit to the author and to Virginia Cooperative Extension, Virginia Tech, and Virginia State University.
Virginia Cooperative Extension is a partnership of Virginia Tech, Virginia State University, the U.S. Department of Agriculture (USDA), and local governments, and is an equal opportunity employer. For the full non-discrimination statement, please visit ext.vt.edu/accessibility.
Publication Date
May 16, 2022



