Phosphorus, Agriculture & The Environment
ID
424-029 (SPES-82NP)
Introduction
Phosphorus (P) is a naturally occurring element that can be found in the earth’s crust, water, and all living organisms. Phosphorus (P) is one of 16 elements that are essential for plant growth. Soils in Virginia are naturally low in phosphorus, and most cropping systems on these soils require supplemental phosphorus to maximize their yield potential. Research has documented that applying fertilizer phosphorus increases crop growth and yields on soils that are naturally low in phosphorus and in soils that have been depleted through crop removal. Crop fertilization represents the greatest use of phosphorus in agriculture today.
Although the economic benefits of phosphorus fertilization on crop production are well documented, too much of a good thing can be detrimental. Excessive soil phosphorus is a potential threat to water quality. The purposes of this bulletin are to increase the understanding of the effect of phosphorus in agricultural systems on water quality and to provide options for efficient economic and environmental management of phosphorus.
Potential Environmental Impacts of Phosphorus
Phosphorus lost from agricultural soils can increase the fertility status of natural waters (eutrophication), which can accelerate the growth of algae and other aquatic plants. Phosphorus is usually the nutrient that controls eutrophication of fresh waters. The USEPA has recommended a limit for controlling eutrophication of 0.05 ppm for total phosphorus in streams that enter lakes and 0.1 ppm for total phosphorus in flowing streams (USEPA, 1986). Acceptable levels of phosphorus in surface runoff from agricultural fields have not been established.
Numerous water quality problems have been associated with eutrophication. Algal blooms can cause fish kills and may harm wildlife and livestock by reducing the oxygen content of water (anoxia) or through the production of toxins. Lakes may become dominated by algae, and coarse, rapidly-growing fish while high value edible fish, submerged macrophytes, and benthic organisms disappear. Eutrophication can result in increased cost and difficulty of drinking water purification. Decaying algal biomass produces surface scums, odors, and increased populations of insect pests.
Phosphorus In Agriculture
Functions of Phosphorus in Plants
In the plant, phosphorus is essential for a number of physiological functions that are involved with energy transformations. Phosphorus is a component of many cell constituents and plays a major role in several key processes, including photosynthesis, respiration, energy storage and transfer, cell division, and cell enlargement. Adequate phosphorus is needed for the promotion of early root formation and growth. Phosphorus also improves crop quality and is necessary for seed formation.
Functions of Phosphorus in Animals
Livestock also require phosphorus for proper growth. In addition to other functions, phosphorus is an essential component of bones and teeth. Animals derive their phosphorus needs from plant products and feed supplements.
Phosphorus and Livestock Operations
The buildup of soil phosphorus to excessive levels can occur when any phosphorus source, including commercial fertilizer, biosolids and manure, is over applied. However, the greatest concern today is with the land application of phosphorus as 3 manure from intensive livestock production. In Virginia, most confined animal feeding operations are primarily located in grain deficient areas and require that grain for animal feed be imported, primarily from the Midwest (Fig. 1). This type of production system represents a net import of nutrients into Virginia due to the combined inputs of phosphorus-enriched feed, dietary feed supplements, fertilizer and other soil amendments. Waste generated from these operations is commonly land applied near the point of generation and commonly within the boundaries of individual farming operations (Fig. 2), which represents a great potential for over applying phosphorus to soil. Primary outputs for phosphorus in this type of system include the crop and animal products that are removed and utilized off the farm (Fig. 2). In most Virginia systems containing confined animal feeding operations, nutrient output leaving the farm boundary will be almost totally in the form of animal products, and the flow of phosphorus into this type of production system is out of balance. Simply put, more phosphorus is entering the farm boundary than is exiting in the form of salable products (Fig. 2).
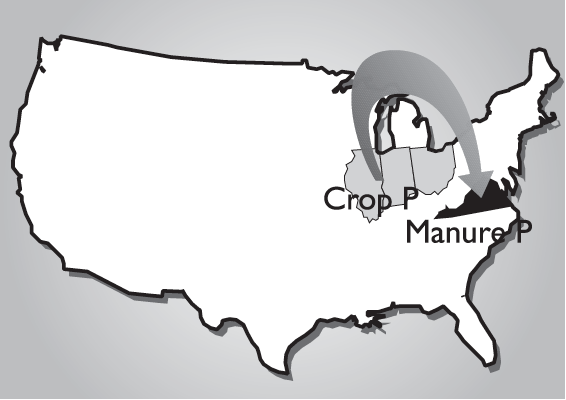
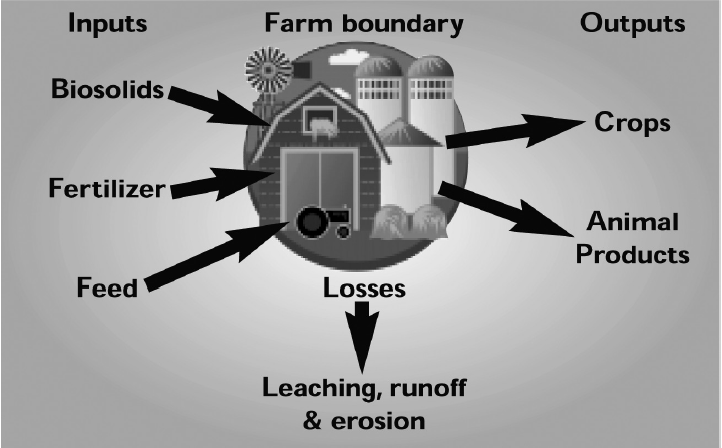
An additional factor that contributes to the over application of phosphorus as manure from poultry and swine is that these animals are not as efficient at utilizing phosphorus in feed as compared to cattle. The diets of mono gastric animals (poultry and swine) are often supplemented by mineral phosphorus additions to provide enough phosphorus for these animals.
Land application of manure to recycle nutrients (Fig. 2) can lead to an accumulation of soil phosphorus, which in turn increases the potential for phosphorus losses by runoff and leaching. A buildup of soil phosphorus in soils treated with animal waste results when manure is applied at rates designed to supply crop nitrogen (N) needs because of the imbalance between the nitrogen and phosphate (P2O5) content of the applied manure as compared to the annual nitrogen and P2O5 requirements of most crops. Most animal manure contains nearly as much P2O5 as nitrogen (Table 1), but plants take up and remove about 2.4 to 4.5 times as much nitrogen as P2O5 (Table 2).
| Manure | Total Nutrient Content (pounds/ton or pounds/1000 gallons)† | ||
|---|---|---|---|
| Nitrogen | P2O5 | K2O | |
| Dry Brolier Litter | 62.58 | 62.12 | 28.57 |
| Dry Turkey Litter | 61.75 | 63.68 | 24.36 |
| Layer or Breeder | 36.46 | 65.06 | 24.22 |
| Liquid Dairy* | 22.61 | 12.07 | 18.92 |
| Semi-Solid Dairy | 10.54 | 6.12 | 8.67 |
| Semi-Solid Beef | 12.79 | 6.67 | 11.30 |
| Swine Lagoon* | 10.14 | 5.68 | 5.72 |
| Mixed Swine* | 41.13 | 29.75 | 18.18 |
* Values are in pounds/1000 gallons. All other values are in pounds/ton.
† Phosphorus and potassium contents of fertilizers and manure are expressed as P2O5 and K2O, respectively, instead of elemental P and K. Conversion between the two forms can be made by knowing that 2.29-pounds of P2O5 is equal to 1-pound of P and that 1.2-pounds of K2O is
| Farm Product | Plant Part | Yield | Nutrient Removal In Harvested Product | ||
|---|---|---|---|---|---|
| N | P2O5 | K2O | |||
| Corn | Grain | 150 bu | 135 | 53 | 40 |
| Stover | 4.5 tons | 100 | 37 | 145 | |
| TOTAL | 235 | 90 | 185 | ||
| Wheat | Grain | 80 bu | 100 | 45 | 49 |
| Straw | 2.0 tons | 34 | 9 | 113 | |
| TOTAL | 134 | 54 | 162 | ||
| Alfalfa | Hay | 4 tons | 180 | 40 | 180 |
| Coastal Bermudagrass | Hay | 8 tons | 60 | 20 | 60 |
| Timothy | Hay | 2.5 tons | 60 | 25 | 95 |
| Beef - Fescue Pasture | 300 lbs. Beef | 9 | 7 | 1 | |
Utilizing Animal Manure on The Basis of its N Versus its P Value: An Example
The imbalance of phosphorus and nitrogen in confined animal feeding operations can be demonstrated by comparing the amount of poultry litter that is required to meet a corn crop’s N needs and P2O5 needs (Table 3). The example illustrated in Table 3 assumes that poultry litter generated from a house with 200 animal units of broilers is applied to corn that is grown on a productive soil with an expected yield of 150 bushels/acre. A crop of 150 bushels of corn would be expected to remove 135 pounds N and 53 pounds P2O5/acre. If applied to supply the expected N needs of the corn, litter would be applied at a rate of 4.2 tons/acre and only 36 acres of crop land would be needed to utilize all of the litter. Applying litter on a N basis provides 215 pounds P2O5/acre above what the corn crop can remove. As a comparison, only 0.83 tons/acre are needed and 181 acres of crop land are needed, if litter is applied at a rate to supply the amount of P2O5 that is removed in the harvested grain. In this P-based system, the corn crop would have a net deficit of 120 pounds N/acre, which would need to be applied as commercial fertilizer. Approximately 5 times more receiving crop land would be needed for the P-based than the N-based system. With the passage of House Bill 1207 by the Virginia General Assembly in January 1999, poultry producers will soon be required to apply poultry litter on a P basis instead of the traditionally used N basis.
Background Information
|
|
Litter Data Total litter = 150 tons/year |
Corn Data Yield = 150 bushels/acre |
| Comparing the methods of Application | |
N Basis Litter rate = 4.2 tons litter/acre |
P2O5 Basic Litter Rate = 0.83 tons litter/acre |
Phosphorus In Agricultural Soils
The Soil Phosphorus Cycle
To understand how to control phosphorus movement and phosphorus losses from soils, one must have a basic understanding of the soil phosphorus cycle. The complex nature of the chemical and microbiological reactions that control phosphorus availability in agricultural systems is illustrated in the soil phosphorus cycle (Fig. 3). Phosphorus in soils originates from the weathering of residual minerals and from phosphorus additions in the form of fertilizers, plant residues, agricultural wastes and/or biosolids. The type of phosphorus bearing minerals that form in soil is highly dependent on soil pH. Phosphorus reacts with iron (Fe) and aluminum (Al) to form insoluble Fe and Al phosphates in acid soils and with calcium (Ca) to form insoluble Ca phosphates in alkaline soils.
The release of soil phosphorus to plant roots and its potential movement to surface waters is controlled by several chemical and biological processes. Phosphorus is released to the soil solution as phosphorus-bearing minerals dissolve, as phosphorus bound to the surface of soil minerals is uncoupled (desorbed), and as soil organic matter decomposes (mineralizes). Most of the phosphorus added to soil as fertilizer and manure is rapidly bound by the soil minerals in chemical forms that are not subject to rapid release; thus, soil solution phosphorus concentrations are typically very low.
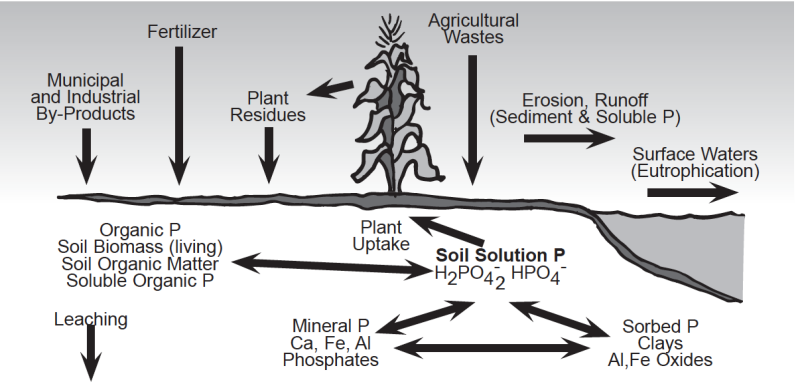
Phosphorus Forms and Amounts in Soil
Plants absorb phosphorus that is dissolved in the soil water (which is referred to as the “soil solution”). Forms of phosphorus dissolved in the soil solution under normal pH conditions include the negatively charged primary orthophosphate anion (H2PO4-) and smaller amounts of the secondary orthophosphate anion (HPO42-).
Total soil phosphorus usually ranges from 800 to 1,600 pounds P (1,800 to 3,700 pounds P2O5) per acre furrow slice (one acre of soil to a depth of six inches and weighing approximately two million pounds); however, most of this phosphorus is unavailable for plant growth because of its low solubility. Phosphorus in the soil solution of most agricultural soils ranges from <0.01 to 1 ppm. An entire acre-furrow slice generally contains less than 0.4 pounds of phosphorus in solution throughout the growing season at any one time; thus, soil solution phosphorus must be replaced on a continuous basis. It is estimated that the soil solution must be replaced up to 300 times during a typical season for crops like corn and soybean. Replenishment of the soil solution occurs due to the release of phosphorus tied up in mineral forms and organic matter (Fig. 3).
Phosphorus Losses & Removal From Agricultural Systems
Phosphorus is removed or lost from the soil by: 1) crop uptake & removal; 2) runoff & erosion; and 3) leaching (Fig. 3). Harvested crops remove phosphorus from the soil and the farm. Phosphorus concentrations in plant tissues typically range from 0.1 to 0.5% on a dry weight basis and most crops utilize or take up between 20 and 90 pounds of P2O5 each year (Table 2). Since soils are natural systems that are constantly subjected to changes due to the combined effects of the environment (i.e., rainfall, weathering, etc.) and management practices, it is impossible to totally eliminate phosphorus losses from soil.
Water moving across the surface or through soils can remove both soluble (dissolved) and particulate (eroded soil particles) forms of soil phosphorus (Fig. 3 & Fig. 4). The transport of particulate and soluble phosphorus can increase the concentration of bioavailable phosphorus in surface waters (i.e., streams, rivers, lakes and oceans). Bioavailable phosphorus is the portion of phosphorus which can be used by aquatic organisms. Phosphorus can also move by leaching, but this mechanism is usually considered less important than surface runoff since phosphorus is held very tightly by soils, especially phosphorus-deficient subsoils. Possible exceptions would include soils with high water tables and phosphorus-saturated soils that would increase the potential for phosphorus leaching.
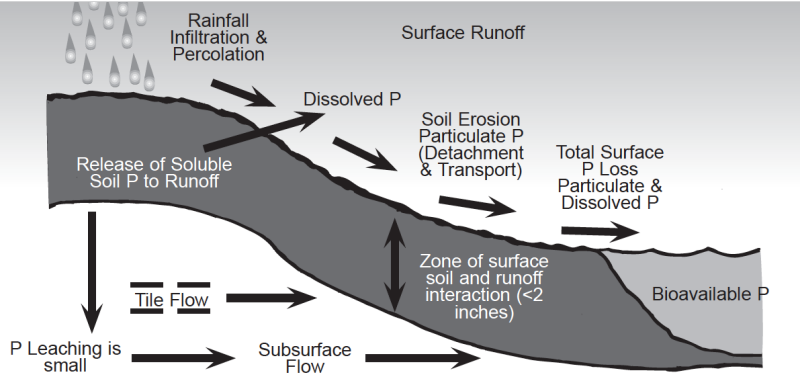
The potential environmental impacts of phosphorus losses from agricultural systems where soil erosion is controlled have been assumed to be low; however, new evidence suggests that runoff losses of dissolved phosphorus from agricultural systems with high soil phosphorus may be a legitimate environmental concern. Soils have a finite capacity to bind phosphorus and desorption of phosphorus can be accelerated, with an increase in runoff phosphorus, when soils become saturated with phosphorus. Thus, if the level of soil phosphorus is allowed to build up by repeated application of phosphorus in excess of crop needs, the soil can become saturated with phosphorus and the potential for losses of soluble phosphorus in surface runoff will increase significantly. Data collected from soils with a history of animal manure applications have shown that soil test phosphorus can be increased dramatically. These new data also suggest that the potential loss of soluble phosphorus in surface runoff will increase with increasing levels of soil test phosphorus. Very high levels of soil test phosphorus can be achieved by over-applying manure, biosolids, and commercial phosphate fertilizer (Fig. 5). Research has also shown that soils will require several years of continuous cropping without phosphorus additions to effectively reduce very high soil test phosphorus levels.
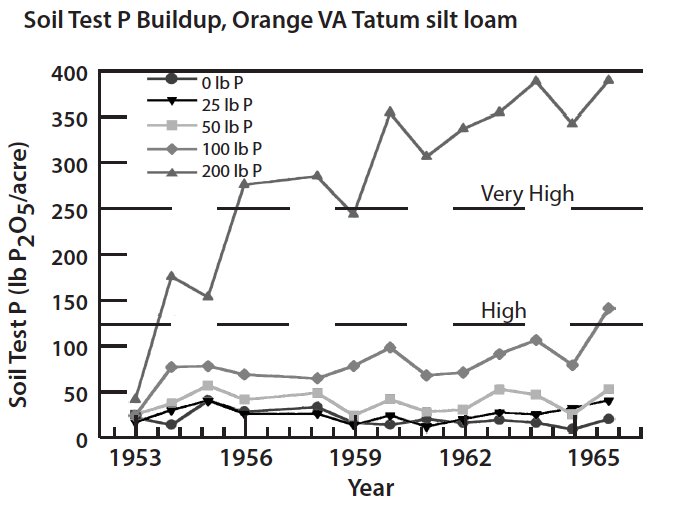
Diagnosing Potential Soil Problems
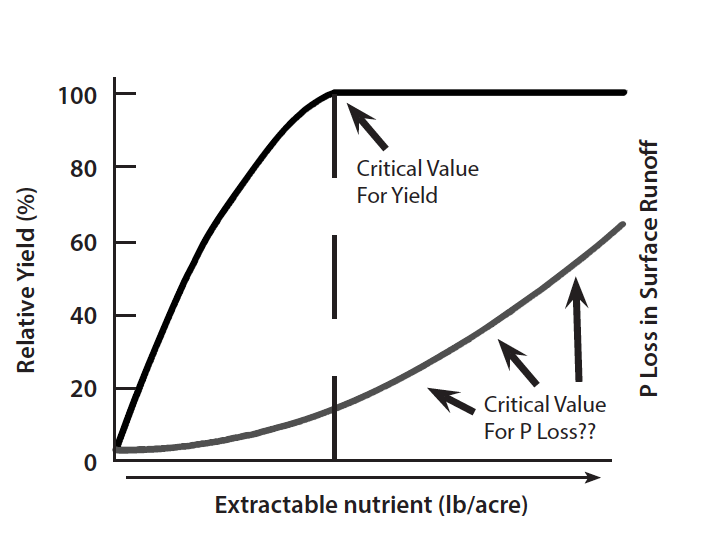
Phosphorus management in today’s agriculture requires the development and implementation of nutrient management tools that maintain profitability while minimizing detrimental environmental impacts. Soil testing has traditionally been used to provide profitable fertilizer recommendations. Soil test correlation permits the amount of a nutrient that is extracted by a specific method to be related to the availability of that nutrient for a given crop. Determining the degree of limitation to crop growth or the probability of obtaining a crop yield response to an applied nutrient at a given soil test level is referred to as soil test calibration. The amount of an extractable nutrient beyond which crop yield no longer increases is called the critical level (Fig. 6). Producers should add enough phosphorus over a period of years to build up its concentration in the soil to the critical level to ensure that yields are not limited by the lack of phosphorus.
It is important to note that soil fertility is only one of several factors that can limit plant yields. Other factors that can affect plant yields include temperature, water supply, incidence of diseases and insects, and poor soil physical conditions. For example, during a very dry growing season, having high soil fertility levels will not necessarily lead to high yields.
When working with phosphorus, it is necessary to distinguish between elemental phosphorus and phosphate (P2O5). This is very important since soil test results may be reported as elemental phosphorus whereas commercial fertilizers are formulated on the basis of phosphate (P2O5). The conversion from an elemental phosphorus to a phosphate basis can be made by knowing that 2.29-pounds of P2O5 is equal to 1-pound of elemental phosphorus. Fertilizer recommendations and animal manure analysis are typically given as the amount of phosphate (P2O5).
Soil test phosphorus gives an indication of the amount of plant available phosphorus in a soil. Soil test extractants dissolve only a small fraction of the total mineral and sorbed soil phosphorus (Fig. 1), which is correlated with expected yield response (Fig. 6). The Virginia Tech Soil Testing Laboratory uses the Mehlich I or dilute double acid extractant for routine soil testing. Since public and private laboratories may use different extractants and procedures, it is important to know which extractant was used before comparing extractable phosphorus levels from different labs. In Virginia, fertilizer recommendations are based on a combination of several factors including routine soil test results, the crop to be grown, and the estimated yield potential of the soil as determined using the Virginia Agronomic Land Use Evaluation System (VALUES; Simpson et al., 1993).
Soil testing is currently considered by many to be the best management tool available to ensure that soils do not accumulate excessive amounts of phosphorus and create situations where soil phosphorus may become an environmental problem. However, research on this topic is incomplete. In this regard, soil testing would not only be used to predict a critical value for maximum crop yield response but also to determine a critical value for acceptable environmental impacts of agricultural phosphorus. In Virginia, the critical value for maximizing crop yields (Fig. 6) is 55 ppm of Mehlich I extractable phosphorus. Soils containing this concentration of soil test phosphorus would not generally be expected to contribute high levels of dissolved phosphorus to surface waters. However, Mehlich I phosphorus levels in excess of 300 ppm have been observed in Virginia soils with a history of poultry litter and/or other manure application.
Phosphorus losses from agricultural systems are affected by site-specific soil and management characteristics (Fig. 4), including type of cropping and tillage system, percent slope, soil type, level of soil test phosphorus, amount and intensity of precipitation, method and timing of fertilizer and manure applications, presence of riparian areas, and other factors. Thus, using the level of soil test phosphorus to predict the potential for environmental impact on surface water quality is more difficult than using it to predict crop yield response. Currently, agronomists and soil scientists are in a dilemma regarding the use of soil testing as a measure of environmentally acceptable levels of soil phosphorus. This dilemma exists because soil test procedures were developed to determine the potential yield response of crops to phosphorus and crop fertilizer needs, not to determine the potential for surface runoff losses of phosphorus. Data recently collected on high phosphorus soils clearly demonstrate that the potential for phosphorus loss in surface runoff increases with increasing levels of soil test phosphorus (Fig. 6). However, there are not enough scientific data to say what level of soil test phosphorus corresponds to a critical value for maintaining surface water quality. Thus, it is important to understand that one cannot use the same calibration to determine crop fertilizer response and predict potential environmental problems from surface runoff. Until newer methods are developed using new information and good science (i.e., a site specific soil Phosphorus Index, etc.), soil testing is still the best management tool available for preventing soils from becoming overloaded with phosphorus.
Practices for Controlling Phosphorus Losses to Surface Waters
Agricultural practices must be implemented that minimize the environmental impacts of phosphorus. Phosphorus enrichment of surface water involves a combination of high soil phosphorus and conditions that favor the transport of phosphorus to surface waters. Thus, reducing soil phosphorus accumulation, runoff, and erosion will decrease the potential for transport of bioavailable phosphorus. Producers should focus on managing both the source of phosphorus and the potential transport of phosphorus out of a field.
- Controlling soil erosion is the critical step in keeping phosphorus in agricultural fields. Soils with high loadings of phosphorus will deliver high loadings of phosphorus to surface streams if sediments are washed into streams. If erosion is controlled, then the potential for losses of dissolved phosphorus in surface runoff becomes the primary concern. Conservation practices that can be used to reduce erosion and surface runoff include strip cropping, conservation tillage, winter cover crops, etc. Filter strips, buffer zones and riparian areas can be effective at trapping sediment phosphorus at field boundaries, but they are not very effective at trapping dissolved phosphorus.
- Nutrient management plans should be prepared for all operations that have confined animal feeding operations. Nutrient management plans are effective tools that can be used to ensure that nutrients, especially phosphorus, are not applied at excessive rates. By October 1, 2001, poultry operations with 200 or more animal units of poultry (20,000 broilers or 11,000 turkeys) will be required by law to have a phosphorus-based nutrient management plan for their farm in Virginia.
- Development and implementation of nutrient management plans by users of organic nutrient sources. A good nutrient management plan is an effective tool that can be used to ensure that all nutrients regardless of source are not applied at excessive rates
- Inject or incorporate fertilizer and organic nutrient sources whenever possible. Injecting or mixing phosphorus with the soil will increase the probability that the applied phosphorus reacts with and becomes bound by the soil, thus reducing its solubility. However, tillage can increase the transport potential of phosphorus by erosion.
- Apply phosphorus and manure to actively growing crops. Avoid manure applications on frozen soils and during the winter when plant growth is limited and runoff potential is high.
- The buildup of soil phosphorus levels should be monitored using routine soil test procedures, and producers should keep good records of their soil test reports over time. Producers should set a goal of not allowing their fields to exceed recommended levels. The current scientific data base does not allow conclusive determination of how much phosphorus can safely accumulate in Virginia soils. Until enough new data are available and site-specific index values of the potential for phosphorus transport can be developed, general soil test guidelines are the best tool for minimizing environmental problems with phosphorus. Producers should remember that it may take several years to reduce soil test phosphorus from very high levels by crop removal after phosphorus additions cease. It is very important to collect soil samples properly, and producers are encouraged to contact their local Virginia Cooperative Extension office for instructions on proper sampling.
- Producers with confined animal feeding operations that do not have sufficient land to utilize all of their manure on farm must consider other options for utilizing excessive manure or increasing the efficiency of utilizing phosphorus in feed. Several options have been proposed and are being considered for utilizing excessive manure and increasing phosphorus use efficiency. These options include:
- Transportation of poultry litter to land areas that need fertilizer phosphorus.
- The use of the phytase enzyme in poultry and swine feed to increase the utilization of phosphorus in feed grain, which reduces the need for supplemental phosphorus in the feed and ultimately reduces the phosphorus content of the manure.
- Composting, pelletization and granulation of manure by combining with commercial fertilizer to produce manure-based fertilizer products that can be marketed off the farm.
- Combustion of dry poultry litter or gasified manure as a source of bioenergy.
- The use of alum in poultry houses has shown promise for improving animal performance as well as lowering the solubility of phosphorus in poultry litter that is land applied.
Conclusion
Water quality is everyone’s concern and it is important for all citizens to remember that soils can become saturated with phosphorus and this excessive soil phosphorus can lead to water quality problems. A long-term goal for agricultural producers, home owners, and others involved in animal and plant production should be to balance the input of nutrients with the movement of nutrients off site, and also to manage soils in ways to minimize phosphorus losses.
References
Donahue, S.J., and S.E. Heckendorn. 1994. Soil test recommendations for Virginia. Virginia Cooperative Extension, Virginia Tech, Blacksburg, VA.
Pierzynski, G.M., J.T. Sims, and G.F. Vance. 1994. Soils and environmental quality. Lewis Publ. Boca Raton, FL. 313 p.
Simpson, T.W., S.J. Donohue, G.W. Hawkins, Margaret M. Monnet, and J.C. Baker. 1993. The development and implementation of the Virginia Agronomic Land Use Evaluation System. Dept. of Crop and Soil Environmental Sciences, Virginia Tech, Blacksburg, VA 83 pgs.
Sims, J.T. (Ed.) 1998. Soil testing for phosphorus: Environmental uses and implications. Southern Cooperative Series Bulletin No. 389.
USDA-SCS. 1994. The Phosphorus Index - A Phosphorus Assessment Tool. Water Resources/Water Quality Technical Note. No. 1901. Soil Conservation Service South National Technical Center.
U.S. Environmental Protection Agency, 1986. Quality criteria for water 1986: Washington, D.C., U.S. Environmental protection Agency Report 440/5-86-001, Office of Water.
Virginia Cooperative Extension. 1987. A handbook of agronomy. VCE Publication 424-100, Virginia Tech, Blacksburg, VA.
Virginia Department of Conservation and Recreation. 1993. Nutrient Management Handbook. Second Edition. Department of Conservation and Recreation, Division of Soil and Water Conservation, Richmond, VA.
Virginia Cooperative Extension materials are available for public use, reprint, or citation without further permission, provided the use includes credit to the author and to Virginia Cooperative Extension, Virginia Tech, and Virginia State University.
Virginia Cooperative Extension is a partnership of Virginia Tech, Virginia State University, the U.S. Department of Agriculture (USDA), and local governments, and is an equal opportunity employer. For the full non-discrimination statement, please visit ext.vt.edu/accessibility.
Publication Date
June 4, 2024



