Common Diseases of Cultured Striped Bass, Morone saxatilis, and Its Hybrid (M. saxitilis x M. chrysops)
ID
600-080 (VM-001P)
EXPERT REVIEWED
Fish Health and Disease
Striped bass (Morone saxitilis) and hybrid striped bass (M. saxitilis x M. chrysops) are widely cultured for both food and sportfishing markets. Because these fish are commonly raised in high densities under intensive aquaculture situations (e.g., cages, ponds, tanks), they are often exposed to suboptimal conditions. Healthy striped bass can generally resist many of the viral, bacterial, fungal, and parasitic pathogens, but the fish become increasingly susceptible to disease agents when immunocompromised as a result of stress.
A number of noninfectious problems are commonly encountered in striped bass and hybrid striped bass culture facilities. Factors such as poor water quality, improper nutrition, and gas supersaturation can directly cause morbidity (clinical disease) and mortality. In addition, any of these conditions can predispose the fish to an infectious disease and, if not properly addressed, can also lead to mortality.
Infectious Diseases
Infectious diseases are a common cause of fish loss in aquaculture, often leading to the demise of the entire fish population. Therefore, an understanding of potential pathogens of striped bass and their hybrids and early disease recognition is critical to prevent significant losses (McAllister, Mann, and McKenzie 1987). It is also important to understand that many disease outbreaks are caused by opportunistic pathogens and thus can be prevented by proper husbandry and management techniques.
Parasites
Parasitic infestations are a common problem in striped bass culture and may have harmful health consequences when fish are heavily parasitized (Smith and Noga 1992).
Ichthyophthiriosis or “Ich” is caused by the ciliated protozoan parasite Ichthyophthirius multifiliis in freshwater or Cryptocaryon irritans in saltwater. These parasites cause raised, white lesions visible on the skin and gill (commonly called “white spot disease”) and can cause high mortalities in a population of fish. The parasite burrows into skin and gill tissue (Figure 1), resulting in osmotic stress and allowing secondary bacterial and fungal infections to become established at the site of penetration. The life cycle of the parasite can be completed in a short time, so light infestations can rapidly progress to severe problems. Because the parasite becomes buried in the tissues of the fish, it is protected from the environment and from the effect of waterborne chemotherapeutic agents.
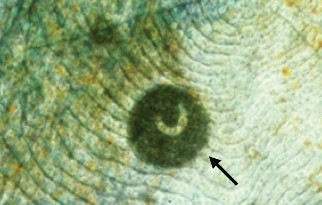
Infestations of Trichodina sp. and Chilodonella sp. in striped bass may cause skin ulceration as well as fin and gill erosion. If these ciliated parasites are present in large numbers, their feeding activity on the surface of the fish can compromise the integrity of the tissues. This often results in osmotic stress and secondary bacterial and fungal infections.
Another ciliated protozoan parasite that commonly causes problems in cultured striped bass is Epistylis sp. (Figure 2). This stalked parasite attaches to the skin, fins, or gills of the fish and feeds on suspended material in the water column. The parasite causes irritation and inflammation at the site of attachment, resulting in tissue erosion and secondary infections. The disease caused by this parasite is often called “red sore disease” due to the multiple small, red hemorrhages on the skin. Large numbers of these parasites can also completely cover the gill tissues, reducing oxygen uptake across the gill surface.
Other protozoan parasites of striped bass are Tetrahymena sp. and Uronema sp. These freshwater and marine parasites, respectively, can burrow directly through intact skin (Figure 3) and migrate to various internal organs. Tissues often have a significant inflammatory reaction, and the normal function of the affected organ can be severely disrupted.

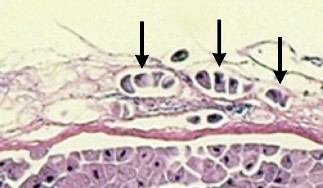
Ichthyobodo sp. are very small tear-shaped flagellated parasites that attach to skin or gill tissue and gain nutrients from the host. Severe infestations and mortalities are often associated with high organic loads in the water and poor husbandry.
The dinoflagellate Amyloodinium ocellatum affects striped bass in brackish and saltwater. This parasite, often called “rust” or “velvet,” can be found on skin and gill tissue where it appears as small, white-to-red-dish spots. Heavy infestations can result in acute mortality due to anoxia and osmotic stress.
Monogeneans are a common external parasite of the skin, fins, or gills of striped bass. These flatworms can cause local irritation at the site of attachment, leading to severe problems when present in high numbers. The presence of these external parasites in a fish population often indicates poor husbandry.
Multiple species of larval digenetic trematodes can invade striped bass tissues (Figure 4). The larval stage of Clinostomum sp., known as the “yellow grub,” generally encysts in the muscle of the fish. The developing parasite may be readily visible, making the fillet commercially undesirable. The life cycle of these digenetic trematodes includes two additional hosts, generally snails and birds. Removal of one of these hosts will interrupt the life cycle of the trematode and eliminate this parasite problem.
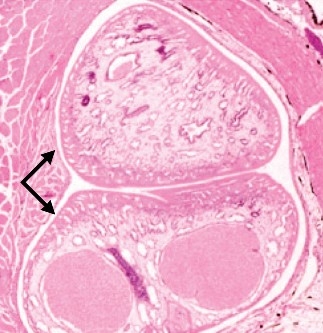
In addition, a number of intestinal parasites – including adult digenetic trematodes, tapeworms, and roundworms – have been reported from wild striped bass. However, these are generally not a serious problem in cultured striped bass or their hybrids.
Bacteria
Most bacterial pathogens of striped bass are ubiquitous in fish and water and may not produce clinical disease. However, when fish become stressed or injured, these infectious agents may cause morbidity and mortality. Furthermore, because striped bass and hybrid striped bass can live in a wide range of salinities, they may be exposed to both freshwater and marine pathogens.
Motile aeromonas septicemia is caused by multiple Aeromonas species. Because these bacteria are ubiquitous in the environment, fish can be at risk of disease at any time. Lesions from this type of infection can include hemorrhage and necrosis of the skin, fins, and internal organs.
Flavobacterium spp. can cause skin and gill lesions on striped bass and their hybrids. A commonly encountered disease is “columnaris,” caused by F. columnaris. This pathogen generally causes problems of the skin, fins, and gills, resulting in pale, ulcerative, necrotic lesions. These lesions are often accompanied by secondary fungal infections. Another bacteria of this group, F. branchiophila, can cause “bacterial gill disease,” resulting in thickening of the gill tissue and hindering oxygen exchange and osmoregulation (Bullock 1990).
Infection with aquatic Mycobacterium spp. can result in a chronic disease in cultured striped bass and their hybrids. Affected fish can exhibit various clinical lesions, including skin ulcerations and erosions, abdominal distention, anorexia (“off feed”) and a generalized chronic wasting syndrome (Figure 5). Granulomas can form in various organs, destroying normal tissues and disrupting organ functions (Figure 6). Infected fish are unsuitable for human consumption. In addition, aquatic species of mycobacteria can be zoonotic pathogens, i.e., capable of causing disease in humans. Thus, destruction of all infected and exposed fish is generally recommended.
Streptococcus iniae can be a serious pathogen of striped bass and other species of fish, most notably tilapia (Shoemaker, Klesius, and Evans 2001). This pathogen causes inflammation of the brain and abdominal cavity as well as necrosis of the liver, spleen, and kidney. The disease can spread quickly through a production facility, and result in high mortalities. This bacterial pathogen is a zoonotic organism.


Edwardsiella tarda occasionally affects striped bass, causing ulcerations and abcesses of the skin, muscle, and internal organs. Often an unpleasant odor accompanies the lesions. This pathogen is also a zoonotic organism and can produce gastrointestinal infection in humans.
Vibriosis is caused by various Vibrio spp. and generally affects fish in brackish and marine environments. Fish become lethargic and develop hemorrhagic, ulcerative lesions on the skin, fins, gills, and eyes. The abdominal cavity often contains bloody fluid from hemorrhagic lesions in the internal organs. Pathogenicity depends on the Vibrio sp. encountered, and mortality rates appear to be influenced by environmental conditions.
The pathogen Photobacterium damsela subspecies piscicida is responsible for “pseudotuberculosis” in striped bass raised in brackish or marine water. Fish become discolored, anorexic, and may develop nodular and hemorrhagic lesions in internal organs. Outbreaks of this disease appear to be closely linked to poor husbandry conditions.
Viruses
Although there are no known viruses that are specifically pathogenic to striped bass or hybrid striped bass, three viral diseases have been described. These viruses can be factors in the overall decline in fish health, making populations more susceptible to other opportunistic infections.
Lymphocystis has been reported in striped bass, although the incidence appears to be infrequent (Krantz 1970). This viral disease does not cause severe pathology or death, but it produces small, raised, nodular lesions on the skin and fins. Fish heal after sloughing infected cells, but the virus released from these cells serves as infectious material that can transmit the disease to other fish. Therefore, affected fish populations should be quarantined to prevent the spread of this disease to susceptible fish.
Striped bass have been reported with infectious pancreatic necrosis virus (IPNV). Clinical signs were limited to darkened pigmentation (Schutz et al. 1984). While the ability of the virus to cause mortality is questionable, IPNV can be transmitted from infected striped bass to other susceptible fish species (McAllister and McAllister 1988). This should be taken into account when undertaking polyculture or stocking striped bass into waters with other susceptible species.
The striped bass aquareovirus can cause hemorrhagic lesions of the skin and swim bladder and enlargement of the liver. However, this virus has not been reported to cause mortality (Baya et al. 1990).
Fungi
Several species of fungi cause infections in striped bass and hybrid striped bass, though only three species are generally considered important.
Saprolegniosis is a fungal disease of fish and fish eggs that is caused by a variety of opportunistic pathogens, including Saprolegnia sp. and Aphanomyces sp. Infections of the skin, fins, or gills occur when fish are debilitated (e.g., injured, diseased, malnourished, environmentally stressed). The pathogen appears on external surfaces as white-to-brown tufts of cottony growth, which are formed by fungal mycelium that grow on fish. Saprolegnia sp. often causes superficial lesions (Figure 7), while Aphanomyces sp. usually cause deeper ulcerative lesions that extend into the muscle (Plumb 1997). Because these fungi are usually opportunistic pathogens, saprolegniosis is commonly associated with bacterial or parasitic infections (Bruno and Wood 1999).

Branchiomyces sp. can infect striped bass, causing “gill rot” (Meyer and Robinson 1973). This disease is generally associated with the culture of fingerlings in water with high temperatures or high organic content. Fungal hyphae invade the tissues of the gill and affected gills become necrotic, leading to impaired oxygen exchange and osmoregulatory deficiency.
Noninfectious Syndromes
Poor water quality, improper nutrition, gas supersaturation, and soft water are noninfectious problems that may result in high morbidity and mortality in cultured striped bass. Poor water quality (e.g., increased ammonia or nitrites) may lead directly to stress, immunosuppression, and gill pathology, which may predispose fish to secondary infections. Diets high in lipid content have been linked to hepatic lipidosis or “fatty liver syndrome,” which may lead to other metabolic problems. Gas supersaturation in fish can result from using water obtained from deep groundwater sources, water with temperature extremes, or recirculated water with air leaks in intake pipes. Supersaturation can cause air bubbles in the skin, fins, and gills, leading to tissue necrosis and chronic stress. Soft water can cause problems in the fish as a result of osmoregulatory imbalance. Morbidity has occurred in striped bass at a water hardness (as calcium carbonate/CaCO3) below 20 parts per million (ppm), and mortalities have occurred with a hardness below 5 ppm (Mitchell 1989). Water hardness between 150-200 ppm is recommended for striped bass and its hybrids.
Diagnostics
As with other fish diseases, the clinical signs for these striped bass diseases are often similar and nonspecific. Because of this, appropriate diagnostic measures should be undertaken. When sick or recently dead fish are given to a veterinarian or fish health professional, a series of diagnostic tests will be used for disease diagnosis. A complete fish necropsy includes visual inspection, skin scrapes, and gill and fin clips for evidence of parasitic, bacterial, or noninfectious diseases. Cultures from external lesions and internal organs should be taken for bacterial isolation and drug sensitivity testing. Impression smears of internal organs can be used to look for signs of parasites or bacteria. Samples can also be taken for more specific tests, such as cell culture and virus isolation. The diagnostic examination is completed with a histopathologic examination that can demonstrate the extent of pathologic changes and confirm the presence of infectious or noninfectious diseases. The clinical history, water-quality parameters, and diagnostic examination results are then interpreted together for a complete picture of the disease. Only then can an appropriate course of therapy, management, and preventive measures be properly designed.
Disease Prevention and Control
Any culture facility should have biosecurity measures in place to prevent the introduction, establishment, or spread of infectious diseases. Pathogens are transmitted among fish through water, feed, fish, equipment, and people. Therefore, water should be treated (e.g., ultraviolet light, ozonation, and/or EPA-approved chemicals) and feed should be purchased from reputable dealers and properly stored. Any fish brought into a facility should be quarantined, tanks should be cleaned and disinfected after removal of a population, and equipment should be disinfected after use. Biosecurity measures for personnel should also be established, using footbaths, protective clothing, and standard operating procedures.
In addition, the establishment of disease in a population of fish can be reduced by the maintenance of a proper environment. For example, poor water-quality parameters, improper temperature, overcrowding, overhandling, and buildup of organic matter can predispose fish to disease. If such factors are eliminated, morbidity and mortality can be often reduced.
References and Additional Reading
Baya, A., A. E. Toranzo, S. Nunez, J. L. Barja, and F. M. Hetrick. 1990. Association of a Moraxella sp. and a reo-like virus with mortalities of striped bass, Morone saxatilis. In Pathology in Marine Science, ed. F. O. Perkins and T. C. Cheng. New York: Academic Press.
Bruno, D. W., and B. P. Wood. 1999. Saprolegnia and other oomycetes. In Viral, Bacterial and Fungal Infections, vol. 3 of Fish Diseases and Disorders, ed. P. T. K. Woo and D. W. Bruno. New York: CABI Publishing, 808.
Bullock, G. L. 1990. Bacterial Gill Disease of Freshwater Fishes. Fish and Wildlife Service Fish Disease Leaflet No. 84. U.S. Department of the Interior. www.lsc.usgs.gov/FHB/leaflets/84.asp.
Krantz, G. E. 1970. Lymphocystis in striped bass, Roccus saxatilis, in Chesapeake Bay. Chesapeake Science 11:137-39.
McAllister, K. W., J. A. Mann, and L. C. McKenzie. 1987. Annotated Bibliography of the Diseases and Parasites of Striped Bass. Fish and Wildlife Service Fish Disease Leaflet No. 76. U.S. Department of the Interior. www.lsc.usgs.gov/FHB/leaflets/76.asp
McAllister, K. W., and P. E. McAllister. 1988. Transmission of infectious pancreatic necrosis virus from carrier striped bass to brook trout. Diseases of Aquatic Organisms 4:101-04.
Meyer, F. P., and J. R. Robinson. 1973. Branchiomycosis: A new fungal disease of North American fishes. The Progressive Fish-Culturist 33:74-77.
Mitchell, A. J. 1989. Parasites and diseases of striped bass. In The Aquaculture of Striped Bass: A Proceeding, ed. J. P. McCraren, 177. College Park, Md.: Maryland Sea Grant.
Paperna, I. and D.E. Zwerner. 1976. Parasites and diseases of striped bass, Morone saxatilis (Walbaum), from the lower Chesapeake Bay. J.Fish Bid. 9: 267-287.
Plumb, J. A. 1997. Infectious diseases of striped bass. In Striped Bass and Other Morone Culture, ed. R. M. Harrell. Amsterdam: Elsevier Science B.V., 271-313.
Schutz, M., E. B. May, J. N. Kraeuter, and F. M. Hetrick. 1984. Isolation of infectious pancreatic necrosis virus from an epitzootic occurring in cultured striped bass, Morone saxitilis (Walbaum). Journal of Fish Diseases 7:505-07.
Shoemaker, C. A., P. H. Klesius, and J. J. Evans. 2001. Prevalence of Streptococcus iniae in tilapia, hybrid striped bass, and channel catfish on commercial fish farms in the United States. American Journal of Veterinary Research 62:174-77.
Smith, S. A., and E. J. Noga. 1992. General parasitology. In Fish Medicine, ed. M. K. Stoskopf. Philadelphia: W.B. Saunders Co., 132-48.
Virginia Cooperative Extension materials are available for public use, reprint, or citation without further permission, provided the use includes credit to the author and to Virginia Cooperative Extension, Virginia Tech, and Virginia State University.
Virginia Cooperative Extension is a partnership of Virginia Tech, Virginia State University, the U.S. Department of Agriculture (USDA), and local governments, and is an equal opportunity employer. For the full non-discrimination statement, please visit ext.vt.edu/accessibility.
Publication Date
June 15, 2020



