Getting Acquainted with Amyloodinium ocellatum
ID
600-200 (CNRE-39P)
EXPERT REVIEWED
What is Amyloodinium ocellatum?
Amyloodinium ocellatum (abbr. A.ocellatum) is a marine dinoflagellate. While most marine dinoflagellates (small protozoan organisms) exist as free living members of the planktonic community, some such as A. ocellatum live at least a portion of their life cycle as parasitic organisms.
What is the life cycle of Amyloodinium ocellatum?
Amyloodinium ocellatum has a simple direct life cycle and exists in its parasitic stage as a trophont (feeding stage), Figure 1. The trophont is attached via anchor-like processes, called rhizoids, and can infest the gills, fins and body of the host fish. When the trophont has matured (average size 80 - 100+ microns), it falls free of the host and forms a tomont, Figure 2. The tomont, an encysted stage which falls to the bottom of the tank or other substrate, subdivides internally and can form as many as 256 infective stages.
These infective stages excyst (hatch) as dinospores (commonly called swarmers), Figure 3, and are the infective stage. These swarmers actively swim through the water column searching for a new host. The duration of this life cycle is temperature dependent, as is trophont size and tomont fecundity, and can range from seven to as many as twenty days.



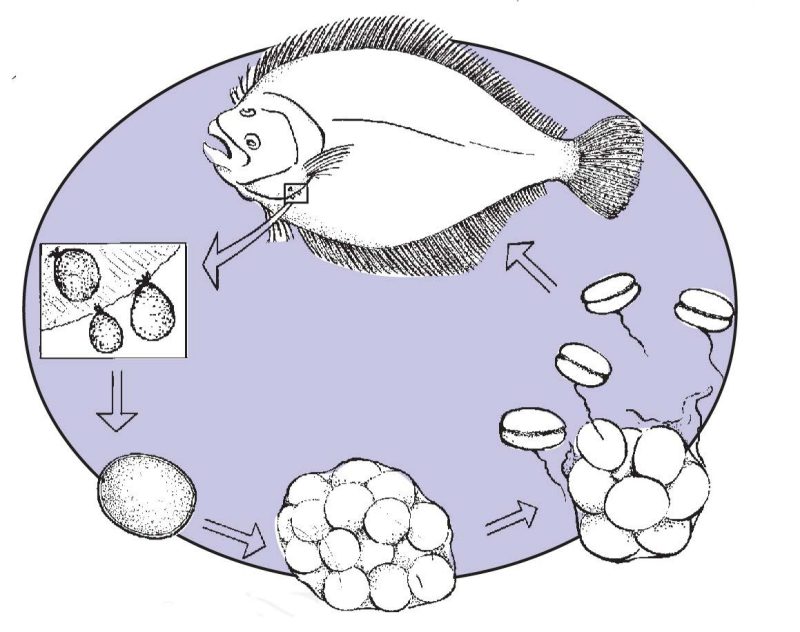
Why is Amyloodinium ocellatum a problem in aquaculture?
Amyloodinium ocellatum causes damage to the host via the rhizoids that penetrate into the epithelial cells of the skin and gills. The parasite obtains nutrition from the contents of the cell and causes degeneration and death of the cell. A. ocellatum’s fecundity rate, wide range of environmental tolerances, and resistance of the trophont and tomont stages to chemotheraputents make early identification of this parasite a high priority in brackish and marine culture systems. Once diagnosed, a quick response is essential to prevent rapid loss of fish stocks.
How can Amyloodinium ocellatum be controlled?
The three main methods by which A. ocellatum can be controlled: chemical treatment, flushing, and filtration. UV irradiation and ozone have also been shown to be effective in reducing numbers of parasites.
Chemical Treatment:
Both formalin (25 mg/L) and hydrogen peroxide (25 mg/L) are FDA approved chemicals available for use as theraputents against this parasite in food fish production. In addition, freshwater dips (3-5 minutes) have been used to reduce the number of trophonts on the gills and skin of the fish. Other chemicals such as copper sulfate, benzalkonium chloride and chloroquine have been used with varying success in ornamental fish. However none of these chemicals are effective at killing the encysted tomont-stage. Thus, in order to completely rid the fish and system of the parasite, repetitive doses every 3 - 10 days (depending upon water temperature) may be required.
Flushing:
Flushing of production systems is another means of reducing infestation levels of A. ocellatum. This is effective by physically removing the encysted tomont stage before the infectious dinospores have the opportunity to excyst. It is important when implementing treatment flushing that the water being removed from the system be withdrawn from the bottom of the culture tank.
Filtration:
One of the better methods to date in the control of A. ocellatum infestations in intensive aquaculture production systems is filtration. This physically removes the tomont stage from the production system while allowing for minimal water exchange. Filtration on a commercial scale in intensive recirculating aquaculture can be accomplished via microscreen, drum or bead filters. While microscreen/drum filters generally have a direct filtrate stream exiting the system, bead filters do not. As such, it is imperative that the bead filters be backwashed a minimum of one time per day. System volume should be filtered at least once per hour, and down to at least fifty microns. In research or hatchery environments, cartridge or diatomaceous earth filters may also be economically employed to control this parasitic disease.
This FACT SHEET was published by:
Virginia Tech
Virginia Sea Grant College Program,
Virginia Cooperative Extension
For further information contact:
Stephen A. Smith
Virginia-Maryland College of Veterinary Medicine
Email: stsmith7@vt.edu


Virginia Cooperative Extension materials are available for public use, reprint, or citation without further permission, provided the use includes credit to the author and to Virginia Cooperative Extension, Virginia Tech, and Virginia State University.
Virginia Cooperative Extension is a partnership of Virginia Tech, Virginia State University, the U.S. Department of Agriculture (USDA), and local governments, and is an equal opportunity employer. For the full non-discrimination statement, please visit ext.vt.edu/accessibility.
Publication Date
July 2, 2024



