Dealing with Trichodina and Trichodina-like species
ID
600-205 (CNRE-38P)
EXPERT REVIEWED
What are Trichodina and Trichodina-like species?
Trichodina spp. are a group of dorsal-ventrally flattened oval ciliated protozoan parasites of marine and freshwater species of finfish. A readily distinguishable characteristic of these organisms is the presence of a prominent denticular or “tooth-like” internal cytoskeleton ring. There are three additional genera of trichodinads (Trichodinella, Paratrichodina and Dipartiella) which are similar in description and life cycle. While small numbers of these organisms on a fish generally do not cause much of a health problem, large numbers can cause moderate to serious pathology and ultimately, death of fish. Small fish and fry are especially susceptible, and mortality can occur quickly if undiagnosed.

How does Trichodina spp. affect the fish?
Trichodina spp. cause damage by feeding on mucus and detritus covering the surface of the gills and skin of the fish causing irritation to the epithelial layer of cells. This can result in hyperplasia (proliferation) of the epithelial cells, clubbing of the gill filaments and even fusion of the gill filaments. This affects the ability of the gills to maintain optimal respiratory and excretory activities, and the ability of the skin to maintain proper homeostatic osmoregulatory properties. Massive infestations of these parasites on fish can also directly result in superficial to deep ulcerative skin lesions which then allow for secondary bacterial and fungal infections to develop at the affected site.
What is the life cycle of Trichodina spp.?
There are species of trichodinads that inhabit freshwater, brackish water and salt water. Some have a preference for the gill area, others for the skin, and others that occur on both the gills and skin of fish. Trichodinads reproduce by simple binary fission under conditions that are usually optimal for the host fish. Most species are host specific and presumably spread from fish to fish by incidental contact between susceptible host fish, as well as through contact with the organism in the water column.

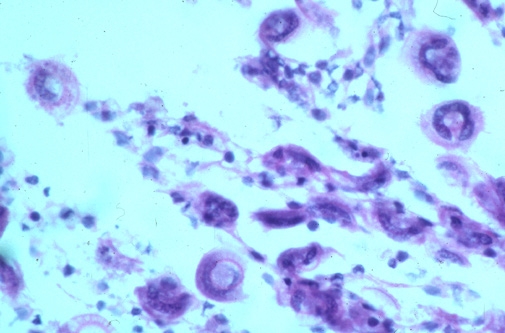
Why is Trichodina spp. a problem in aquaculture?
Trichodina spp can cause extensive fish mortality in an aquaculture system. The ability of this parasite to quickly multiply under certain environmental conditions or when the fish are stressed by other factors makes early detection of this parasite a high priority in an aquaculture facility. Once diagnosed, an appropriate treatment or management response is essential to prevent rapid loss of fish stocks.
How can Trichodina spp. be controlled?
There are several methods by which Trichodina spp. may be controlled in the aquaculture of foodfish. These include chemical treatments, freshwater baths, and flushing. UV is generally considered ineffective due to the high dosage rates required to kill the organism.
Chemical Treatment
The only FDA-approved chemical for the treatment of external parasites on foodfish is aquaculture-approved formalin. This is probably the best method to date for controlling Trichodina spp. infestations in an aquaculture system. A formalin bath of 170-250 ppm for 60 minutes is the FDA-approved recommendation. However, experience has shown that a single formalin bath may not completely remove all of the parasites from fish, especially marine fish, and long term or periodic treatments may be needed to keep this parasite under control. Therefore a continuous bath of 25 ppm formalin is also approved by the FDA for use on foodfish. This may affect the biofiltration of a recirculation system slightly, but usually only for a short period of time. This dosage may be repeated as necessary based upon periodic monitoring until no Trichodina spp. are detectable via skin scrapes. In addition, sodium chloride (salt), regarded as a Compound of Low Regulatory Priority by the FDA, may be used at 1.5-3.0 ppt to treat Trichodina spp. infestations on freshwater fish.
Water Bath
Another common method for controlling Trichodina spp. on marine finfish is to utilize periodic fresh water dips. Though stressful on fish due to increased handling and the osmotic stress, this method can be very effective in reducing the overall number of parasites on fish. This is an effective method for treating individual fish such as broodstock, but may not be a viable option in a production facility due to the logistics associated with handling and treating large numbers of fish.
Flushing
Flushing of production systems (i.e., the removal of system water prior to treatment) is another means of reducing infestation levels of Trichodina spp. This method may be effective by physically removing any dislodged parasites in the water column from the system.
UV Treatment
Recently, UV irradiation treatment of the water column has been examined as a potential control method for infections caused by ectoparasitic protozoans. A UV dose of 2.2 x 106 μW s/cm2 of the circulating rearing water succeeded in control of Trichodina truttae infection in juvenile salmon (Oncorhynchus keta).
References
1. Gaze, W.H. and R. Wootten. 1998. Ectoparasitic species of the genus Trichodina (Ciliophora:Peritrichida) parasitizing British freshwater fish. Folia Parasitologica 45:177-190.
2. Lom, J. 1995. Protozoan and Metazoan Infections. In Fish Diseases and Disorders, Volume 1. P.T.K. Woo, ed. CABI Publishing, New York, NY.
3. Mizuno S., S. Urawa, M. Miyamoto, M. Hatakeyama, N. Koide and H. Ueda. 2018. Ectoparasitic protozoans Ichthyobodo salmonis and Trichodina truttae in juvenile chum salmon using ultraviolet disinfection of rearing water. J Fish Dis. 2018:1–12.
4. Smith, S.A. and E.J. Noga. 1992. General Parasitology. In Fish Medicine. M.K. Stoskopf, ed. W.B. Saunders Co. Philadelphia, PA.
5. Wellborn, T.L. 1967. Trichodina (Ciliata: Urceolariidae) of Freshwater Fishes of the Southeastern United States. J. Protozool. 14:399-412.
This fact sheet was published by:
Virginia Tech, the Virginia Sea Grant College Program, and Virginia Cooperative Extension
Photos: Stephen Smith, DVM, PhD
For further information contact:
Stephen A. Smith
Virginia-Maryland College of Veterinary Medicine
stsmith7@vt.edu
This publication is the result of research supported by the NOAA Office of Sea Grant, the U.S. Department of Commerce, and the Virginia Sea Grant College Program.


Virginia Cooperative Extension materials are available for public use, reprint, or citation without further permission, provided the use includes credit to the author and to Virginia Cooperative Extension, Virginia Tech, and Virginia State University.
Virginia Cooperative Extension is a partnership of Virginia Tech, Virginia State University, the U.S. Department of Agriculture (USDA), and local governments, and is an equal opportunity employer. For the full non-discrimination statement, please visit ext.vt.edu/accessibility.
Publication Date
July 2, 2024



