Lime Calibration for Soilless Media- A Tool for Greenhouse and Nursery Producers
ID
SPES-718NP
Introduction
Rootzone pH is important to manage to produce marketable nursery and greenhouse stock. This is primarily because mineral nutrients are more available under certain conditions (within specific pH levels).
Soilless substrates are essentially inert and contain little nutritional value. Thus, the producer is responsible for supplementing nearly all applied mineral nutrients and water for proper plant development. This goes beyond simple fertilization, where maintaining optimal rootzone pH levels is critical to ensure that applied nutrients are available to the plant. If not, plants can exhibit symptoms of nutrient deficiency or toxicity, resulting in delayed or decreased yield or reduced quality.
The first step in ensuring that rootzone conditions are healthy for the plant is gauging the current pH status of the rootzone and correcting it through proper lime adjustments. This extension article highlights the importance of rootzone pH in soilless substrates and explains how to adjust rootzone conditions prior to production by performing lime calibrations.
What is pH, and why is it Important?
pH is a measure of the concentration of active hydrogen ions (H+). But what does that mean? The greater concentration of hydrogen ions signifies more acidic pH levels, or lower pH, whereas a lower concentration of hydrogen ions signifies more basic pH levels, or higher pH.
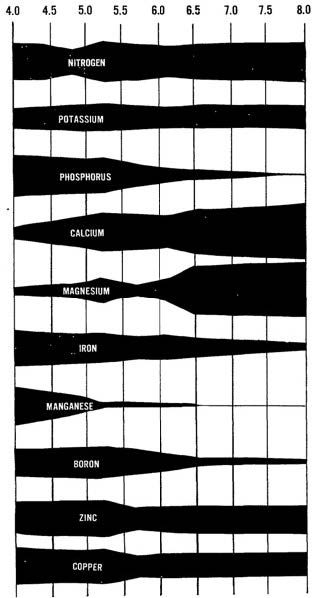
pH is a critical substrate chemistry metric that determines the mineral nutrient availability, as well as nutrient toxicities. As shown in figure 1, the solubility of mineral nutrients changes with different pH levels. The optimal pH range for most most nutrients are available, although this can vary by species.
For example, calcium is most readily available between pH levels of 6.0 and 7.5. The rootzone can have large amounts of calcium ions; however, if the pH is 4.0, the calcium is less available to the plant and is not as usable.
Soilless Substrates
Relative to mineral soils (i.e., sand, silt, or clay), soilless substrates are significantly more porous with less particle surface area. As a result, there are limited amounts of exchange sites (where hydrogen or other ions can bind). Some soilless substrates (those with a greater surface area and finer materials) typically have more sites and require higher application rates to saturate or fill these sites. The soilless substrates used most in mass container production, peat- or bark-based media, are inherently acidic. Thus, soilless substrates are either purchased pre-mixed with some amount of lime, or lime needs to be added to achieve the desired pH.
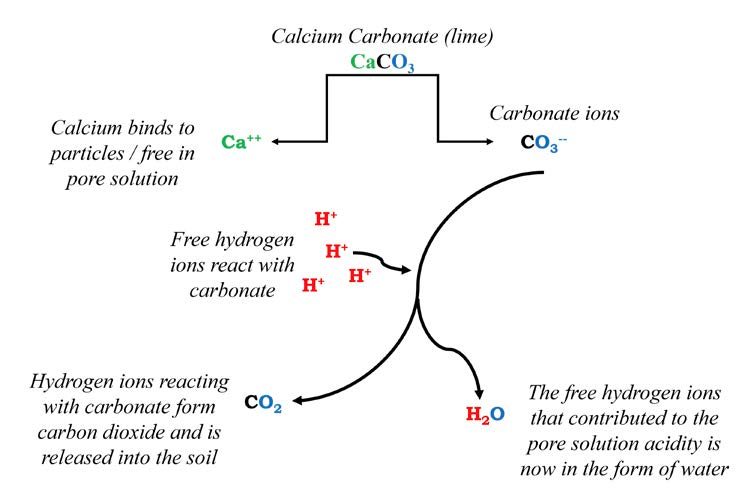
Increasing substrate pH is commonly achieved through lime applications. Two popular lime types are calcitic (calcium carbonate; CaCO3) or dolomitic [dolomite; (CaMg(CO3)2)] lime, and they come in different particle sizes. A pulverized or finer grade is usually faster acting and will activate more quickly. Both are relatively cheap and commercially available. Figure 2 illustrates how lime materials help increase pH.
Peat-based media
In greenhouse operations, peat-based media is the primary substrate composite used in the U.S. Typically, but not always, peat composites are pre- conditioned before packaging and shipping.
Specifically, it is common for peat suppliers to amend the composite with wetting agents, starter fertilizer charge, and a lime solution (for raising pH values). In this case, rootzone pH is already corrected.
In unamended peat bales, pH levels are typically 3.5-4.5 and require lime amendments. However, there are different grades of peat, from particle size to age, that can change lime rates.
Bark-based media
Most post-processed (hammermilled and screened) pine bark is acidic (3.0-4.5) and often needs a pH correction before transplanting. While a standard pine bark substrate is typically screened with a ½” screen, the coarseness of the material will significantly influence the lime rate applied, since finer bark materials have more surface area and may require greater lime rates.
Measuring Substrate pH
A fast and effective way to gauge substrate fertility and measure pH is by using a pH probe (commercially available online or at garden centers).
Substrate Measurement Extract
A substrate measurement extract (SME) measures the pH of the substrate material, not the pH of the pore solution. This method is also referred to as a 1:1 or 2:1 method (1 part substrate to 1 part deionized or distilled water).
It entails placing a known volume of substrate material in a container and applying the same (1:1) or half the volume (2:1) of deionized or distilled water. After applying the water, mix the substrate and water with a mixing stick and wait for approximately five minutes (fig. 3). This waiting period is designed to detach the H+ ions from the particle surface. Simply stick the pH probe in the cup to measure the pH.

Lime Calibration
The challenge in applying lime is determining the correct amount to apply when a targeted pH level is desired. This is particularly important when a producer is growing a variety of crops that have different pH requirements or sensitivities. Measuring how much lime to apply or to target a certain pH can be ascertained by conducting a lime calibration.
To do this, you’ll need the following items below (fig. 4):
- pH probe
- Plastic cups with units
- Scale
- Sealable plastic bags
- Lime
- Deionized or distilled water
- Soilless media
- Conduct an SME on your soilless media before lime amendments and record the values and the date.
- Do this on at least three separate samples.
- A manageable sample size is 200 mL of media, or 0.8 cups.
- Place approx. 2 liters (or ~½ gallon) of your hydrated media in a sealable or zipper plastic bag.
- To create a strong calibration curve, fill six separate bags with each media tested.
- Lime rates are typically measured using pounds per cubic yard. Since we are working on a small scale, and depending on what units your scale outputs, you can use:
- Grams per cubic liter
- Ounces/pounds per cubic gallon
- If you are using 2 liters or ½ gallon, calculate 2-, 4-, 6-, 8-, and 10 lbs. per cubic yard of lime and set aside:
- Grams per cubic liter (lbs. yd-3 x 0.593)
- 0 lbs. yd-3 = 0.0 g L-3
- 2 lbs. yd-3 = 1.2 g L-3
- 4 lbs. yd-3 = 2.4 g L-3
- 6 lbs. yd-3 = 3.6 g L-3
- 8 lbs. yd-3 = 4.7 g L-3
- 10 lbs. yd-3 = 5.9 g L-3
- Ounce per cubic gallon (lbs. yd-3 x 0.079)
- 0 lbs. yd-3 = 0.00 oz gal-1
- 2 lbs. yd-3 = 0.16 oz gal-1
- 4 lbs. yd-3 = 0.32 oz gal-1
- 6 lbs. yd-3 = 0.48 oz gal-1
- 8 lbs. yd-3 = 0.63 oz gal-1
- 10 lbs. yd-3 = 0.79 oz gal-1
- Amend your bagged media with the calculated lime rate, seal the bag, and agitate (mix or shake) very well. If the media isn’t well hydrated, pour some regular tap water to hydrate and mix.
- Every 3 days, for 12 days (resulting in 5 total measurements), repeat step #1.
- Do not place the tested sample back into the bag (i.e., discard).
- Be sure to rinse off the pH probe with distilled water after each measurement.
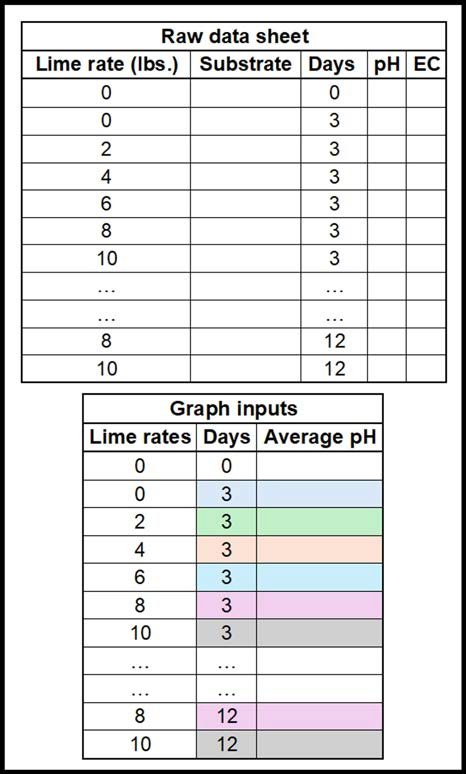
- Plot your data in Microsoft Excel and create the calibration curve.
- View the data sheet example in (fig. 5) below for formatting.
- You will need to select the average pH values by using ‘= average( )’ equation within each lime rate and days data points.
- To load the graph:
- Click ‘insert chart’ > ‘insert scatter with straight lines and markers.’
- Right-click on the graph and select ‘Select data’
- Each lime rate will be considered a series. On the left, click ‘Add series.’ Label the series name as the lime rate.
- Your x-values will be ‘Days’, and your y- values will be the ‘Average pH’.
- After 12 days, which pH is your target? Amend your media at this rate.
- Each line will resemble a different lime rate.
Assessing Fertility During Production
Rootzone conditions can significantly change during the production cycle, influenced by factors such as irrigation water quality (alkalinity, hardness), the type of fertilizer applied (slow-release, fertigation, organic, or synthetic), and the stage of plant growth. After your lime calibration, it is recommended that rootzone fertility is periodically checked to ensure that rootzone conditions are still healthy for the plant.
SMEs
As previously described, growers can gauge pH values by frequently measuring their substrate pH (every 3-4 weeks) by conducting SMEs from the substrate surface. This can provide insight into whether modifying fertility programs is needed.
Pore Solution
Other methods for indirectly assessing rootzone fertility can be evaluated by conducting a pore through analysis (Wright, 1986) or a tilt test. This entails displacing the pore solution out of the container or collecting the pore solution near the base of the container. Growers can use a pH probe to measure both substrate pH and electrical conductivity (soluble salts) in the pore solution.
Leaf Samples
If you are noticing deficiency or toxicity symptoms across plants, and conducting a SME or pore through did not reveal anything telling, consider sending in leaf samples to a lab for analysis. Unfortunately, the Virginia Tech Plant Disease Clinic does not provide a plant tissue analysis. Below is a partial list of regional laboratories:
- North Carolina Dept of Agriculture (https://www.ncagr.gov/divisions/agronomic- services/plant-tissue-analysis) (Raleigh, NC)
- Penn State Agricultural Analytical Services Laboratory (https://agsci.psu.edu/aasl) (State College, PA)
- Louisiana State University AgCenter (https://www.lsuagcenter.com/portals/our_of fices/departments/spess/servicelabs/soil_testi ng_lab) (Baton Rouge, LA)
Conclusions
Ensuring optimal pH levels in soilless rootzones is critical for producing high-quality crops. Conducting lime calibration curves and targeting pH levels before potting and transplanting can serve as a powerful tool for growers. Additionally, it can mitigate downstream challenges related to fertility issues affecting plant quality and yield.
Although lime adjustments can be easier and more effective before transplant, if you need to increase your rootzone pH post-transplant, read HERE (https://www.canr.msu.edu/news/commonly_used_li mestones_for_adjusting_ph_in_greenhouse_mixes) on some available options.
Additional Resources
Southern Nursery Association’s Best Management Practice Manual. 2013. https://ellisonchair.tamu.edu/2013/12/29/best- management-practices-guide-for-producing-nursery- crops/
LeBude, A. and T. Bilderback. 2009. The Pour- Through Extraction Procedure: A Nutrient Management Tool for Nursery Crops. NC State University Extension Center. AG-717. https://content.ces.ncsu.edu/the-pour-through- extraction-procedure-a-nutrient-management-tool- for-nursery-crops
Acknowledgements
The author would like to thank Dr. Eric Stallknecht for his time and efforts reviewing this article.
References
Wright, R.D. 1986. The pour-through nutrient extraction procedure. HortScience 21:227-229.
Virginia Cooperative Extension materials are available for public use, reprint, or citation without further permission, provided the use includes credit to the author and to Virginia Cooperative Extension, Virginia Tech, and Virginia State University.
Virginia Cooperative Extension is a partnership of Virginia Tech, Virginia State University, the U.S. Department of Agriculture (USDA), and local governments, and is an equal opportunity employer. For the full non-discrimination statement, please visit ext.vt.edu/accessibility.
Publication Date
July 23, 2025



