Laboratory Procedures: Virginia Tech Soil Testing Laboratory
ID
452-881 (SPES-91P)



Introduction
Most of the procedures for soil analysis used in the Soil Testing Laboratory were established in the early 1950s*. Although the chemical principles have not changed, procedures have been revised over the years to utilize advances in instrumentation which allow more accurate and rapid chemical determinations.
*Rich, C.I., 1955. Rapid soil testing procedures used at Virginia Polytechnic Institute. Virginia Agriculture Experiment Station. Bull.475, p. 8.
A routine test, consisting of eleven analyses, is performed on all samples. In addition, two separate tests are offered on a request basis. These tests are applicable only under certain conditions for which research and calibration work has been conducted. The routine and special tests consist of the following:
Routine Test
soil/water pH (WpH)
buffer index/ pH (BpH)
phosphorus (P)
potassium (K)
calcium (Ca)
magnesium (Mg)
zinc (Zn)
manganese (Mn)
copper (Cu)
iron (Fe)
boron (B)
Special Tests
soluble salts
organic matter
Sample Preparation
Soil samples arrive in 1/2-pint cardboard cartons. Generally, Soil Sample Information Sheets (SSIS) are packaged with the samples. The cartons are opened in a separate preparation area and placed in drying trays. Twenty-eight unknown samples plus two control samples are placed in each drying tray. The two control samples are one known internal reference sample and either a blank or replicate sample. At this time, each sample is assigned a laboratory number which, along with the year, is stamped on the SSIS. The samples are numbered consecutively each calendar year, beginning with 1 on January 1.
The trays of samples are placed in a cross-flow forced-air drying cabinet through which room-temperature filtered air is drawn. The air can be heated 5° to 8°C above the ambient temperature for drying wet samples. Samples remain in the drying cabinet over two nights.
Air-dried (at 20° to 40°C) samples are crushed with a stainless steel hammer mill-type crushing machine and passed through a 10-mesh (2-mm opening) stain-less steel sieve. The samples are then returned to the original sample boxes until the various subsamples are measured out.
Water pH (WpH) Determination
Buffer Solutions: Color-coded buffer solutions of pH 4.0, 7.0, and 10.0 are purchased from commercial sources.
Electrode Internal Filling Solution: Use Thermo Orion’s 3 M KCl, (with no silver), Ross™ Sure-Flow® Internal Filling Solution, Cat. No. 810007.
Procedure:
Daily, do a two-point calibration of the pH meter using fresh buffer solutions of pH 4 and 7, and ensure the calibration before starting every batch of samples.
Scoop 10 cm3 of soil from the prepared sample into a 50-ml beaker. With an automatic pipetting machine add 10 ml of distilled water for a 1:1 (vol/vol) ratio. Thoroughly mix the solution with a glass/plastic rod or mechanical stirrer and allow it to sit for a minimum of 10 minutes and a maximum of 2 hours.
The automated pH analyzer is set to stir solutions for a 5-second equilibration delay before starting to take pH readings. It then continues to stir the soil suspension while the software waits for 10 readings to be stable within 0.02 pH units. Probes are automatically washed after a pH reading greater than 7.5 or less than 4.5. Readings are electronically recorded to the 0.01 pH unit. The pH readings of quality-control soil samples are manually checked before uploading the sample data to verify that they are within current expected values.
Notes:
- For fine-textured soils containing a high level of organic matter, it may be necessary to add an additional 10 mlof distilled water to make a suspension.
- The TPS pH meter has a temperature sensor for automatic temperature compensation (ATC). This ATC probe should sit in a flask of ambient temperature water within the LabFit pH Analyser next to the soil samples being measured.
- If a pH probe’s reading becomes sluggish, unstable, or not reproducible (possibly indicating that the liquid reference junction has become clogged), depress the electrode’s top cap to flush the junction.
Buffer Index/ pH (BpH) Determination
Mehlich Buffer Preparation:
Using a 4-liter volumetric flask, add:
~ 2 liters of distilled water (DW);
10 ml of glacial acetic acid, CH3COOH, 99.5%, 17.4N;
39 ml of 50% triethanolamine (1 TEA : 1 DW);
72.0 g of sodium glycerophosphate, hydrate, C3H5(OH)2PO4Na2·xH2O, FW=216.04(anhy.); or 1,2,3-Propanetiol mono (dihydrogen phosphate) disodium salt, (HOCH2)2CHOPO3Na2; or Glycerol phosphate Disodium salt Hydrate, C3H7O6PNa2, CAS #: 154804-51-0 or 1555-56-2 for alpha structure {Gallard-Schlesinger’s 50 kg GSODGLYERO via Doe & Ingalls, or City Chemical’s 2.5 kg S8040, or Sigma’s 1 kg G 6501};
172.0 g of ammonium chloride (NH4Cl); 48.0 g of calcium chloride dihydrate (CaCl2· 2H2O); {or alternatively use 80.0 g BaCl2· 2H2O}.
Stir using a stir-bar and stir-plate until all salts are dissolved and allow the solution to warm up to room temperature.
Bring to the 4-liter volume with distilled water.
Adjust to pH 6.60 ±0.04 when diluted 1:1 with distilled water.
Use drops of acetic acid to lower the pH or drops of 1:1 aqueous TEA to raise the pH. Use an acid standard to check the preparation of the buffer mixture as follows: combine 10 ml of buffer, 10 ml of distilled water, and 10 ml of commercially prepared 0.05N HCl solution. This mixture should drop the initial buffer pH by 1.40±0.1 units. If the pH is not within these limits, check the preparation of the buffer reagent to make certain that all ingredients were added properly.
Make only what will be needed for a week to prevent microbial growth in storage.
Procedure:
On samples with a WpH ≤6.94, add 10 ±0.2 ml of the Mehlich buffer solution using the 1:1 (vol/vol) soil-water mix from the water pH determination. Thoroughly mix the solution with a glass/plastic rod and allow it to sit for a minimum of 30 minutes. Stir the solution again immediately before reading and while the pH probe is equilibrating in the soil suspension. Record the first stable pH reading to the nearest 0.01 unit. Verify calibration of pH electrodes before measuring buffer pH’s. Check the pH of the buffer solution on the daily blank sample. A rise in its pH indicates fungal growth in the buffer.
Determination of P, K, Ca, Mg, Zn, Mn, Cu, Fe, B, and Al
Extracting Solution (Mehlich 1, 0.05N HCl in 0.025N H2SO4):
Measure approximately 15 liters of distilled water into a 20-liter plastic container. Add 14.0 ml of concentrated sulfuric acid (H2SO4), 82.0 ml of concentrated hydrochloric acid (HCl), and distilled water to make a 20-liter volume and mix thoroughly.
Extraction Procedure:
Measure one 4-cm3 scoop of prepared soil into a 60-ml straight-walled plastic extracting beaker, and add 20 ml of the Mehlich 1 extracting solution with an automatic pipetting machine. The samples are shaken on a reciprocating shaker with a stroke length of 3.8 cm for 5 minutes at 180 oscillations per minute and filtered through Whatman No. 2 (or equivalent), 11-cm filter paper soon after the shaking stops.
Analysis Procedure:
All elements are analyzed in the same extract by an ICP (inductively coupled plasma atomic emission spectrometer). Transfer filtrate from the extraction beaker to an ICP autosampler cup by using a disposable polyethylene pipette. The transfer is a two-step procedure with the first aliquot being a rinse and the second aliquot for the actual transfer. Pipette 4 ml of filtrate and discard into a waste beaker. Pipette another 4 ml of the same filtrate into the autosampler rack’s polystyrene sample cups.
Once all sample filtrates have been transferred, cover the autosampler rack with plastic wrap to prevent air-borne contaminants (dust, lint, etc.) from getting into the solutions. This is important to prevent ICP nebulizer clogging and contamination.
Samples may be stored overnight by covering them with plastic wrap, parafilm, or capping and placing them in a refrigerator. After refrigeration, allow the samples to equilibrate to room temperature before ICP analysis.
Elemental Analysis by ICP:
An ICP instrument, equipped with an autosampler, is set up to analyze 30 samples for 10 elements in about 20 minutes. Each sample has a 24 second preflush with a 10 second integration time to read the element and background spectral lines, and there is approximately a 10 second rinse that mainly occurs during the integration time. A quality control solution is read and verified after every tray of 30 samples.
ICP Working Standards:
The ICP is calibrated with the following series of standards (Note: atomic absorption standards are not sufficiently pure for ICP standards; use only spectrally pure, plasma-quality standards).
Soil #1: Final solution concentration: 0.05 N HCl and 0.025 N H2SO4.
Use the Mehlich 1 (M1) extracting solution or to approximately 250 ml of deionized water in a half-liter volumetric flask, add 2 ml of concentrated reagent grade HCl, and 0.35 ml of concentrated reagent grade H2SO4, dilute to volume with deionized water and mix well.
Soil #2: Final elemental concentration in solution: 30 μg ml-1 P, 2 μg ml-1 Zn, 2 μg ml-1 B.
To approximately 250 ml of M1 extracting solution in a half-liter volumetric flask, add 15 ml of 1000 μg ml-1 P calibration standard, 1 ml of 1000 μg ml-1 Zn calibration standard, 1 ml of 1000 μg ml-1 B calibration standard and dilute to volume with extracting solution and mix.
Soil #3: Final elemental concentration in solution: 300 μg ml-1 Ca, 100 μg ml-1 K, 50 μg ml-1 Mg, 10 μg ml-1 Al, 10 μg ml-1 Mn.
Add to a half-liter volumetric flask with approximately 250 ml of M1 extracting solution 15 ml of 10,000 μg ml-1 Ca calibration standard, 5 ml of 10,000 μg ml-1 K calibration standard, 2.5 ml of 10,000 μg ml-1 Mg calibration standard, 5 ml of 1,000 μg ml-1 Al calibration standard, and 5 ml of 1000 μg ml-1 Mn calibration standard; dilute to volume with extracting solution and mix.
Soil #4: Final elemental concentration in solution: 10 μg ml-1 Cu, 25 μg ml-1 Fe.
Add to a half-liter volumetric flask with approximately 250 ml of M1 extracting solution 5 ml of 1000 μg ml-1 Cu calibration standard and 12.5 ml of 1000 μg ml-1 Fe calibration standard; dilute to volume with extracting solution and mix.
ICP Quality Control Standard:
The quality control solution is prepared with spectrally pure, ICP-quality, calibration stock solutions. (Note: For the elements P, K, Ca, and Mg, use standard stock solutions from a manufacturing source other than the one used to pre-pare the working standards.) Add to a half-liter volumetric flask with approximately 250 ml of Mehlich 1 extracting solution the following amounts of each stock solution then dilute to volume with extracting solution and mix well:
| Element | Final Concentration (µg ml-1) | High Purity Reference Solution |
|---|---|---|
| P | 10 | 5 ml of 1,000 µg ml-1 |
| K | 30 | 1.5 ml of 10,000 µg ml-1 |
| Ca | 200 | 10 ml of 10,000 µg ml-1 |
| Mg | 20 | 1 ml of 10,000 µg ml-1 |
| Zn | 1 | 0.5 ml of 1,000 µg ml-1 |
| Mn | 1 | 0.5 ml of 1,000 µg ml-1 |
| Cu | 1 | 0.5 ml of 1,000 µg ml-1 |
| Fe | 5 | 2.5 ml of 1,000 µg ml-1 |
| B | 1 | 0.5 ml of 1,000 µg ml-1 |
Calculation of Elemental Concentrations:
For each element, the calculation for ppm in soil is as follows:
ppm in solution x 5 = ppm in soil on a volume basis (mg/dm3)
ppm in solution x 4 = ppm in soil on a weight basis (mg/kg)
where 4 is the dilution factor assuming a soil scoop density of 1.25 g/cm3.
ppm values on lab reports are on a weight basis
To convert from ppm (wt. basis) to lbs/acre the equation is:
ppm in soil x 2 = lbs/acre
where weight of an acre furrow slice (6 2/3-inch depth) is assumed to be 2 million pounds.
Estimation of CEC by Summation
Theory:
The Cation Exchange Capacity (CEC) can be reasonably estimated by summation of the Mehlich 1 extractable bases, or non-acid generating cations (Ca, Mg and K), plus the acidity estimated from the Mehlich soil-buffer pH after conversion of all analytical results to meq/100 cm3 or cmol(+)/kg.
This calculated method is closer to an Effective CEC, which is measured at the present pH of the soil, than it is to the soil’s potential CEC, which is measured in solutions buffered at pH 7.0 or higher.
This method is inappropriate for soils with a high soluble salts level or for alkaline soils because these soils may be over-fertilized, calcareous, gypsiferous, or relatively unweathered and could result in an erroneously high CEC value by the release of nonexchangeable cations.
Calculation:
Estimated Soil CEC = Acidity + Ca + Mg + K (in the units of meq/100 g soil or cmol/kg)
Acidity (meq/100 g of soil) = 37.94 - (5.928 x BpH)
where BpH = Mehlich soil-buffer pH reading for an individual soil sample.
meq Ca/100 g = lb Ca per Acre ÷ 401
meq Mg/100 g = lb Mg per Acre ÷ 243
meq K/100 g = lb K per Acre ÷ 782
Sodium is not included in the equations since it is not routinely determined in the Mehlich 1 extract in routine analysis. Since exchangeable Na is usually at a very low concentration, its omission is not considered to be a cause of error in the calculated CEC. If sodium was included, then the calculation would be meq Na/100 g = lb Na per Acre ÷ 460.
The commonly used unit of meq/100 g is equivalent to the SI accepted unit of cmol/kg. 1 meq/100 g = 1 cmol(+)/kg
Soluble Salts
Conductivity Standard:
Use a commercially prepared NIST traceable conductivity standard of 1,000 or 1,420 µsiemens/cm.
or
Prepare potassium chloride standard solution (0.01 N KCl): Dissolve 0.7456 g of potassium chloride (KCl) in deionized water in a 1-liter volumetric flask. Mix well and dilute to volume. The conductivity of this solution at 25°C is 1,412 μsiemens/cm.
Procedure:
Measure one 20-cm3 scoop of prepared soil into a 50-ml beaker, add 40 ml of distilled water for a soil: water ratio of 1:2 (vol/vol). Include at least one internal soil reference (“test”) sample per batch of unknown soil samples. Stir the solution and allow the suspension to settle for at least 1 hour. Check the conductivity meter’s calibration against the conductivity standard. At 25°C, the standard has an electrical conductivity of 1.00 or 1.41 mmho/cm (or mS/cm). Set the meter in the Temperature Compensation Conductivity mode, and cell constant (C) to 1.00/cm. The electrical conductivity (EC) of the supernatant liquid of the soil-water solution is determined with the meter set on the µS/cm scale. Use the bulb to draw the supernatant into the cell. Dispose of this aliquot into a waste beaker. Draw a second aliquot of the sample into the cell and when the meter stabilizes, record the EC as one tenth of the meter’s reading, (move the decimal one place to the left on the meter’s display), in order to give the results in mhos x 10-5 units. The ppm soluble salts in the soil are calculated from the following equation:
ppm soluble salts in soil = EC x 6.4 x 2
In this equation, EC represents the conductivity reading in mhos x 10-5, 6.4 is the factor for converting the conductivity measurement to ppm soluble salts, and 2 represents the water volume dilution factor. Report as ppm soluble salts in soil.
Useful Equations:
EC (mho x 10-5/cm) / 100 = mmho/cm
ppm (mg salt/liter) / 1280 = mmho/cm
0.1 S/m = 1 dS/m = 1 mS/cm = 1 mmho/cm
Resistance of a solution is the reciprocal of the electrical conductivity; therefore,
0.1 µmho = 10.0 Mohm.
Soil Organic Matter (SOM) by Walkley-Black (WB)
Reagent A: Sodium dichromate solution (0.67M): Dissolve 500 g of reagent grade sodium dichromate (Na2Cr2O7 • 2H2O) in tap water to a volume of 2 1/2 liters.
Reagent B: Concentrated reagent grade sulfuric acid (H2SO4).
Procedure:
The procedure is a modified Walkley-Black method. Measure one 1.5-cm3 scoop of prepared soil into a 200-ml test tube. Under a hood, add 20 ml of Reagent A to the soil followed by 20 ml of Reagent B. Allow the solution to cool at least 40 minutes. After cooling, add 100 ml of tap water, mix the solution, and allow to stand overnight (or at least 8 hours). After incubation, withdraw an aliquot of the supernatant using a syringe-type pipette and transfer it to a colorimeter vial. Take readings using a colorimeter set to a 645 nm wavelength. The percentage of organic matter is determined by reference to the following table.
| Colorimeter Reading |
Organic Matter, % |
Colorimeter Reading |
Organic Matter, % |
Colorimeter Reading |
Organic Matter, % |
|---|---|---|---|---|---|
| 100 | 0.0 | 56 | 2.6 | 30 | 6.4 |
| 99-95 | 0.1 | 55 | 2.7 | 29 | 6.6 |
| 94-91 | 0.2 | 54 | 2.8 | 28 | 6.8 |
| 90-88 | 0.3 | 53 | 2.9 | 27 | 7.0 |
| 87-86 | 0.4 | 52 | 3.0 | 26 | 7.2 |
| 85 | 0.5 | 51 | 3.1 | 25 | 7.4 |
| 84-83 | 0.6 | 50 | 3.2 | 24 | 7.6 |
| 82 | 0.7 | 49 | 3.3 | 23 | 7.8 |
| 81-80 | 0.8 | 48 | 3.4 | 22 | 8.0 |
| 79 | 0.9 | 47 | 3.5 | 21 | 8.3 |
| 78-77 | 1.0 | 46 | 3.6 | 20 | 8.7 |
| 76 | 1.1 | 45 | 3.7 | 19 | 9.0 |
| 75-74 | 1.2 | 44 | 3.8 | 18 | 9.4 |
| 73 | 1.3 | 43 | 3.9 | 17 | 9.7 |
| 72-71 | 1.4 | 42 | 4.0 | 16 | 10.1 |
| 70 | 1.5 | 41 | 4.2 | 15 | 10.4 |
| 69-68 | 1.6 | 40 | 4.4 | 14 | 10.8 |
| 67 | 1.7 | 39 | 4.6 | 13 | 11.1 |
| 66-65 | 1.8 | 38 | 4.8 | 12 | 11.5 |
| 64 | 1.9 | 37 | 5.0 | 11 | 11.8 |
| 63-62 | 2.0 | 36 | 5.2 | 10 | 12.2 |
| 61 | 2.1 | 35 | 5.4 | 9 | 12.5 |
| 60 | 2.2 | 34 | 5.6 | 8 | 13.0 |
| 59 | 2.3 | 33 | 5.8 | 7 | 13.5 |
| 58 | 2.4 | 32 | 6.0 | 6 | 14.0 |
| 57 | 2.5 | 31 | 6.2 | 5-1 | 15.0 |
Soil Organic Matter (SOM) by Weight Loss On Ignition (LOI)
Procedure:
Tare balance and weigh 50-mL beakers. Scoop 5 cm3 of air-dried, 2-mm sieved soil into a beaker. Dry for a minimum of two hours at 150°C ±5°C. Maintain at 100°C until weighing. Record the weight of the beaker plus the warm soil sample to ±1 mg. Heat at 360°C for two hours after the temperature reaches 360°C ±5°C. Cool to 105°C and maintain at 105°C until weighing. Weigh the beaker and warm ash in a draft-free environment to ±1 mg. Calculate and report %LOI as percent organic matter to the nearest tenth of a percent.
Calculations:
Dried Soil (Soild) = (Wt of Beaker + Wt of Soil at 150°C) - Wt of Beaker
Ashed Soil (Soila) = (Wt of Beaker + Wt of Soil at 360°C) - Wt of Beaker
Percent weight loss on ignition (%LOI):
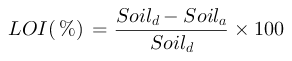
Note:
The LOI (a gravimetric, dry oxidation) method is used to estimate the soil organic matter content for all samples except for those coming from commercial farmland in the Piedmont counties of Virginia. The Walkley-Black (a wet, chemical oxidation) method is used in those cases, due to the presence of gibbsite (Al2O3 • 3H2O) in the clay fraction of soil material in that area of the state. Gibbsite has been reported to lose substantial amounts of water at around 300°C.
Instruments for Soil Analyses
| Analysis | Instrument |
|---|---|
| Soil Drying | Cross-flow forced-air soil drying cabinet, developed at Virginia Tech |
| Soil Grinding | Agvise soil grinder |
| pH Auto-analyzer | LabFit Pty Ltd, model AS-3010D Automated Dual pH Analyser |
| pH Meter | TPS Pty Ltd, model WP-80D, Dual pH-mV and temp. meter |
| pH Electrode | FisherbrandTM accuTupH Rugged Bulb pH Combination Electrode with BNC connector, Cat. No. 13-620-183A |
| Nutrient Extraction | Eberbach Reciprocating, Variable Speed Shaker No. 6000 |
| Elemental Analysis of P, K, Ca, Mg, Zn, Mn, Cu, Fe, B & Al | ICP-AES (Inductively Coupled Plasma - Atomic Emission Spectrometer), ARCOS model with a SOP (radial) view of the plasma, made by Spectro Analytical Instruments and equipped with a CETAC ASX560 autosampler. |
| Soluble Salts | YSI 3100 Conductivity Instrument with a YSI 3254 Pyrex 5-ml Fill Cell |
| Organic Matter - WB | Thermo Scientific Genesys 20 Colorimeter |
| Organic Matter – LOI | Blue M Electric High Temperature (up to 704°C), Ul- tra-Temp, forced-air drying oven, model CW-6680F, with Pro 550 microprocessor-based controller. |
| Organic Matter – LOI | XSR1203S Mettler Toledo (MT) analytical balance with MT’s BalanceLink software (v4.0.2). |
ICP Parameters
The ICP is housed in an instrument room maintained at 21°C (70°F) ± 1°C (2°F). Extreme swings in both temperature and humidity can affect the analytical results. Solutions are introduced to an OptiMist nebulizer and cyclonic spray chamber with a peristaltic pump.
The following analytical lines are used:
| Element | Wavelength (nm) |
|---|---|
| P | 178.287 |
| K | 766.491 |
| Ca | 373.690 |
| Mg | 279.079 |
| Zn | 213.856 |
| Mn | 257.610 |
| Cu | 324.754 |
| Fe | 259.940 |
| B | 249.678 |
| Al | 308.215 |
References
Sample Preparation:
Gelderman, R.H., and A.P. Mallarino. 1998. Soil Sample Preparation. p. 5-6. In Brown, J.R. (ed.). Recommended Chemical Soil Test Procedures for the North Central Region. North Central Regional Research Publication Bull. No. 221 (revised). Missouri Agricultural Experiment Station SB 1001, University of Missouri, Columbia, Mo.
Hoskins, B. and D. Ross. 1995. Soil Sample Preparation and Extraction. p. 3-10. In Sims, J.T. and A.M. Wolf (ed.). Recommended Soil Testing Procedures for the Northeastern United States. Northeastern Regional Pub. No. 493 (2nd edition). Agricultural Experiment Station University of Delaware, Newark, Del.
pH:
Kalra, Y.P. 1995. Determination of pH of soils by dif- ferent methods: collaborative study. Journal of the Association Off. Analytical Chemistry International 78(2):310-321.
Mehlich, A. 1976. New Buffer pH Method for Rapid Estimation of Exchangeable Acidity and Lime Requirement of Soils. 7(7):637-653.
Soil Analysis Handbook of Reference Methods. 1999. Buffer pH and Lime Requirement. p. 41-55. Soil and Plant Analysis Council, Inc., Athens, Ga.
Soil Analysis Handbook of Reference Methods. 1999. Soil pH, and Exchangeable Acidity and Aluminum. p. 27-39. Soil and Plant Analysis Council, Inc., Athens, Ga.
Phosphorus:
Kuo, S. 1996. Phosphorus. p. 869-919. In D.L. Sparks (ed.) Methods of Soil Analysis. Part 3. Chemical Methods. Soil Science Society of America Book Ser. 5. SSSA and ASA, Madison, Wis.
Murphy, J. and J. P. Riley. 1962. A modified single solu- tion method for the determination of phosphate in natu- ral waters. Anal. Chim. Acta. 27:31-36.
Soil Analysis Handbook of Reference Methods. 1999. Phosphorus. p. 69-91. Soil and Plant Analysis Council, Inc., Athens, Ga.
Potassium, Calcium, Magnesium:
Mehlich, A. 1953. Determination of P, Ca, Mg, K, Na, and NH4. North Carolina Soil Test Division (Mimeo. 1953).
Soil Analysis Handbook of Reference Methods. 1999. Major Cations. p. 93-115. Soil and Plant Analysis Council, Inc., Athens, Ga.
Zinc:
Alley, M.M., D.C. Martens, M.G. Schnappinger, Jr., and G.W. Hawkins. 1972. Field calibration of soil tests for available zinc. Soil Science Society of America Proceedings 36:621-624.
Manganese:
Cox, F. R. 1968. Development of a yield response pre- diction and manganese soil test interpretation for soy- beans. Agronomy Journal 60:521-524.
Organic Matter:
Combs, S.M. and M.V. Nathan. 1998. Soil Organic Matter. p. 53-58. In Brown, J.R. (ed.) Recommended Chemical Soil Test Procedures for the North Central Region. North Central Regional Research Publication Bull. No. 221 (revised). Missouri Agricultural Experiment Station SB 1001, Univ. of Missouri, Columbia, Mo.
Method 2.7.08. Chapter 2. p. 37. In MCuniff, P.A. (ed). Official Methods of Analysis of AOAC International, 16th edition. AOAC, Inc., Arlington, Va.
Nelson, D.W., and L.E. Sommers. 1996. Total Carbon, Organic Carbon, and Organic Matter. p. 961-1010. In
D.L. Sparks (ed.) Methods of Soil Analysis. Part 3. Chemical Methods. Soil Science Society of America Book Ser. 5. SSSA and ASA, Madison, Wis.
Peech, M., L.T. Alexander, L.A. Dean, and J. Fielding Reed. 1947. Methods of soil analysis for soil-fertility investigations. USDA Circ. 757, p. 5-7.
Schulte, E.E. 1995. Recommended Soil Organic Matter Tests. p. 52-60. In Sims, J.T. and A.M. Wolf (ed.). Recommended Soil Testing Procedures for the Northeastern United States. Northeastern Regional Pub. No. 493 (2nd edition). Agricultural Experiment Station Univ. of Delaware, Newark, Del.
Schulte, E.E. and B.G. Hopkins. 1996. Estimation of Soil Organic Matter by Weight Loss-On-Ignition. p. 21-31. In Magdoff, F.R., M.A Tabatasbai, and E.A. Hanlon, Jr. (ed.) Soil Organic Matter: Analysis and Interpretation. SSSA, Inc., Madison, Wis. Special Pub. No. 46; Proceedings of a symposium sponsored by Divisions S-4 and S-8 of the Soil Science Society of America in Seattle, Wash. 14 Nov. 1994.
Schulte, E.E., C. Kaufmann, and J.B. Peter. 1991. The influence of sample size and heating time on soil weight loss-on-ignition. Comm. In Soil Science and Plant Analysis 22(1-2):159-168.
CEC by Summation:
Hajek, B. F., F. Adams, and J. T. Cope. 1972. Rapid determination of exchangeable bases, acidity, and base saturation for soil characterization. Soil Science Society of America Proceedings. 36:436-438.
Isaac, Robert A., and William C. Johnson. 1984. Methodology for the Analysis of Soil, Plant, Feed, Water and Fertilizer Samples (revised). University of Georgia, Athens, Ga.
Sumner, M.E., and W.P. Miller. 1996. Cation Exchange Capacity and Exchange Coefficients. p. 1221-1222. In
D. L. Sparks (ed.) Methods of Soil Analysis. Part 3. Chemical Methods. Soil Science Society of America Book Ser. 5. SSSA and ASA, Madison, Wis.
Warncke, D., and J.R. Brown. 1998. Potassium and Other Basic Cations. p. 33. In Brown, J.R. (ed.) Recommended Chemical Soil Test Procedures for the North Central Region. North Central Regional Research Publication Bull. No. 221 (revised). Missouri Agricultural Experiment Station SB 1001, Univ. of Missouri, Columbia, Mo.
Wolf, Ann and Douglas Beegle. 1995. Recommended Soil Tests for Macronutrients: Phosphorus, Potassium, Calcium and Magnesium. Chapter 5. Cation Exchange Capacity section. p. 31. In Sims, J.T., and A.M. Wolf (ed.). Recommended Soil Testing Procedures for the Northeastern United States. Northeastern Regional Pub. No. 493 (2nd edition). Agricultural Experiment Station Univ. of Delaware, Newark, Del.
Soluble Salts:
Rhoades, J. D. 1996. Salinity: Electrical Conductivity and Total Dissolved Solids. p. 417-435. In D. L. Sparks (ed.) Methods of Soil Analysis. Soil Science Society of
America Book Ser. 5 Part 3. Chemical Methods. SSSA and ASA, Madison, Wis.
Soil Analysis Handbook of Reference Methods. 1999. Conductance, Soluble Salts, and Sodicity. p. 57-67. Soil and Plant Analysis Council, Inc., Athens, Ga.
U.S. Salinity Laboratory Staff. 1954. Determination of the properties of saline and alkali soils. Chapter 2. p. 7-33. In L. A. Richards (ed.) Diagnosis and improve- ment of saline and alkali soils. Agriculture Handbook No. 60. USDA-ARS.
Waters, W.E., W. Llewelyn, C.M. Geraldson, and S.S. Woltz. 1973. The interpretation of soluble salt proce- dures as influenced by salinity testing procedure and soil media. Proceedings Tropical Region American Society of Horticulture Science. 17:397-405.
Whitney D.A. 1998. Soil Salinity. p. 59-60. In Brown,
J.R. (ed.) Recommended Chemical Soil Test Procedures for the North Central Region. North Central Regional Research Publication Bull. No. 221 (revised). Missouri Agricultural Experiment Station SB 1001, Univ. of Missouri, Columbia, Mo.
Instrumentation:
APHA-AWWA-WEF. 1998. Standard Methods for the Examination of Water and Wastewater. 20th ed. Section 3120, p. 3:37-43. In Clesceri, L.S., A.E. Greenberg, and
A.D. Eaton (eds.) American Public Health Association, Washington, D.C.
Soil Analysis Handbook of Reference Methods. 1999. Methods of Instrumental Analysis. p. 207-224. Soil and Plant Analysis Council, Inc., Athens, Ga.
Soltanpour, P.N., G.W. Johnson, S.M. Workman, J.B. Jones Jr., and R.O. Miller. 1996. Inductively Coupled Plasma Emission Spectrometry. p. 91-139. In D.L. Sparks (ed.) Methods of Soil Analysis. Part 3. Chemical Methods. Soil Science Society of America Book Ser. 5. SSSA and ASA, Madison, Wis.
Walsh, L. M. 1971. Instrumental Methods for the Analysis of Soils and Plant Tissue. Soil Science Society of America, Inc., Madison, Wis.
Watson, M.E. and R.A. Isaac. 1990. Analytical Instruments for Soil and Plant Analysis. p. 691-740. In R.L. Westerman (ed.) Soil Testing and Plant Analysis. 3rd ed. Soil Science Society of America Book Ser. 3. SSSA, Inc., Madison, Wis.
Websites for Regional Soil Testing Procedures and Other Related Procedures:
Burt, R. (ed.). 2014. Kellogg Soil Survey Laboratory Methods Manual. Soil Survey Investigations Report No. 42 Version 4.0. USDA- NRCS, Lincoln, Nebr. Available at
http://www.nrcs.usda.gov/wps/portal/nrcs/main/soils/research/guide/ (verified 14 Dec. 2018).
Donohue S.J. (ed.). 1992. Reference Soil and Media Diagnostic Procedures for the Southern Region of the United States [Online]. Southern Coop. Ser. Bull. No. 374. Virginia Agricultural Experiment Station, Virginia Tech, Blacksburg, Va. Available at
http://aesl.ces.uga.edu/sera6/PUB/bulletinNo374.asp (verified 14 Dec. 2018)
Northeast Coordination Committee on Soil Testing (NEC-1012). 2011. Recommended Soil Testing Procedures for the Northeastern United States. Northeastern Regional Pub. No. 493 (3rd edition). Agricultural Experiment Station Univ. of Delaware, Newark, DE. Available at
http://extension.udel.edu/lawngarden/soil-health-composting/recommended-soil-testing-procedures-for-the-northeastern-united-states/ (verified 14 Dec. 2018).
Recommended Chemical Soil Test Procedures for the North Central Region. 2015 North Central Regional Research Publication Bull. No. 221 (revised). Missouri Agricultural Experiment Station SB 1001, University of Missouri, Columbia, Mo. Available at
https://extension2.missouri.edu/SB1001 (verified 27 Feb. 2018)
Richards, L.A. (ed.). 1954. Diagnosis and Improvement of Saline and Alkali Soils. Agriculture Handbook No. 60. USDA-ARS. Available at
http://www.ars.usda.gov/Services/docs.htm?docid=10158 (posted 20 Oct 2010).
Savoy H.J. (ed.). 2007. Procedures Used by State Soil Testing Laboratories in the Southern Region of the United States. Southern Coop. Ser. Bull. #190-D. Clemson Experiment Station, Clemson, SC Available at https://ag.tennessee.edu/spp/SPP%20Publications/SCSBno190.pdf (verified 13 Feb. 2019).
Sikora, F.J. and K.P. Moore (ed.). 2014. Soil Test Methods from the Southeastern United States. Southern Coop. Ser. Bull. No. 419. Southern Extension and Research Activity Information Exchange Group - 6 (SERA-IEG-6), Available at http://aesl.ces.uga.edu/sera6/PUB/ MethodsManualFinalSERA6.asp (verified 14 Dec. 2018).
Virginia Cooperative Extension materials are available for public use, reprint, or citation without further permission, provided the use includes credit to the author and to Virginia Cooperative Extension, Virginia Tech, and Virginia State University.
Virginia Cooperative Extension is a partnership of Virginia Tech, Virginia State University, the U.S. Department of Agriculture (USDA), and local governments, and is an equal opportunity employer. For the full non-discrimination statement, please visit ext.vt.edu/accessibility.
Publication Date
March 5, 2024



