Denitrifying Bioreactors: An Emerging Best Management Practice to Improve Water Quality
ID
BSE-55P (BSE-354P)
EXPERT REVIEWED
What is a Denitrifying Bioreactor?
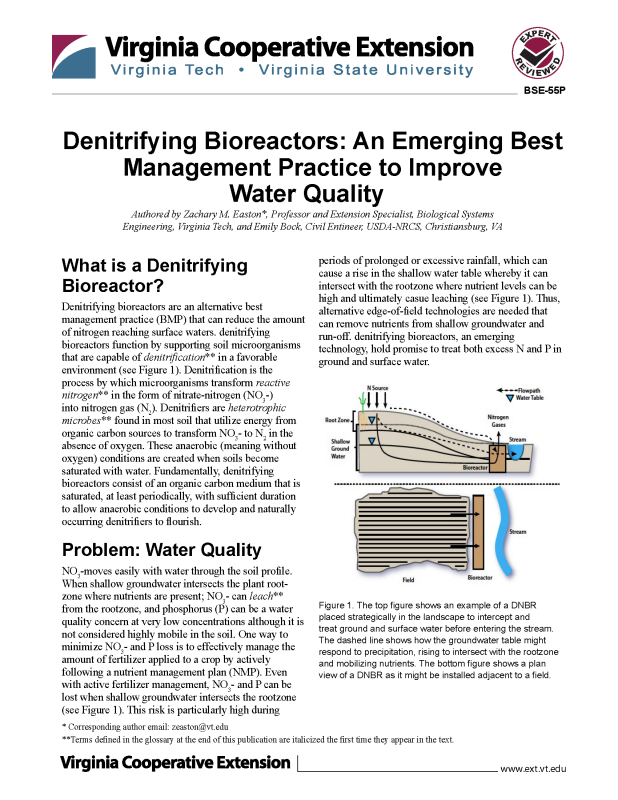
Denitrifying bioreactors are an alternative best management practice (BMP) that can reduce the amount of nitrogen reaching surface waters. Denitrifying bioreactors function by supporting soil microorganisms that are capable of denitrification** in a favorable environment (see Figure 1). Denitrification is the process by which microorganisms transform reactive nitrogen** in the form of nitrate-nitrogen (NO -) into nitrogen gas (N ). (see Figure 1). This risk is particularly high during periods of prolonged or excessive rainfall, which can cause a rise in the shallow water table whereby it can intersect with the rootzone where nutrient levels can be high and ultimately cause leaching (see Figure 1). Thus, alternative edge-of-field technologies are needed that can remove nutrients from shallow groundwater and run-off. Denitrifying bioreactors, an emerging technology, hold promise to treat both excess N and P in ground and surface water. Denitrifiers are heterotrophic microbes** found in most soil that utilize energy from organic carbon sources to transform NO3- to N2 in the absence of oxygen. These anaerobic (meaning without oxygen) conditions are created when soils become saturated with water. Fundamentally, denitrifying bioreactors consist of an organic carbon medium that is saturated, at least periodically, with sufficient duration to allow anaerobic conditions to develop and naturally occurring denitrifiers to flourish.
Problem: Water Quality
NO3- moves easily with water through the soil profile. When shallow groundwater intersects the plant root- zone where nutrients are present; NO3- can leach** from the rootzone, and phosphorus (P) can be a water quality concern at very low concentrations although it is not considered highly mobile in the soil. One way to minimize NO3- and P loss is to effectively manage the amount of fertilizer applied to a crop by actively following a nutrient management plan (NMP). Even with active fertilizer management, NO3- and P can be lost when shallow groundwater intersects the rootzone (see Figure 1). This risk is particularly high during periods of prolonged or excessive rainfall, which can cause a rise in the shallow water table whereby it can intersect with the rootzone where nutrient levels can be high and ultimately cause leaching (see Figure 1). Thus, alternative edge-of-field technologies are needed that can remove nutrients from shallow groundwater and runoff. Denitrifying bioreactors, an emerging technology, hold promise to treat both excess N and P in ground and surface water.
Denitrification is important because it is the only permanent removal of bioavailable** nitrogen from an ecosystem. Even relatively low N concentrations in receiving water bodies can cause eutrophication** and damage fisheries. The U.S. Environmental Protection Agency (EPA) recommends that the maximum stream nitrate-N concentration be less than 0.3 parts per million (ppm) for the Coastal Plain region. Higher levels of NO3-, particularly in drinking water, can lead to infant toxicity (methemoglobinemia or Blue Baby Syndrome) or formation of carcinogenic compounds. The EPA has set a maximum contaminant level (MCL)** for nitrate- N in drinking water at 10 ppm.
Applications
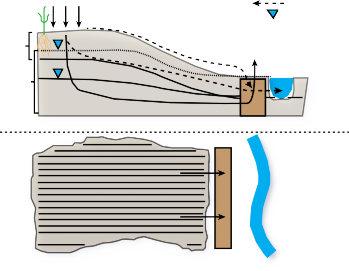
Denitrifying bioreactors have been used to treat a range of nitrate-laden waters including greenhouse effluent, contaminated groundwater, septic system plumes, domestic wastewater, and agricultural runoff. Common designs include walls intercepting shallow groundwater (as in Figures 1 and 2), reactor vessels that receive tile drainage from agricultural fields, beds where the influent is piped in, and streambed bioreactors. The different designs are adapted and employed in the various settings. Many types of organic carbon have been tested for use in denitrifying bioreactors, but woodchips are the most widely used because of their superior hydraulic properties and general availability in larger quantities.
Research has shown that successful nitrogen removal can be obtained in these field scale** systems for up to 15 years even with fluctuating influent nitrate concentrations and flow rates. This tolerance to variable influent enables application of denitrifying bioreactors to treat a wide range of non-point source pollution,** such as that created by agriculture, where conventional wastewater treatment is cost-prohibitive. Some of the greatest potential for denitrifying bioreactor use is in agricultural settings, where nitrogen loss to groundwater is the dominant pathway.
Current Research
The denitrification wall denitrifying bioreactor receiving shallow groundwater and surface runoff from agricultural land at the Eastern Shore AREC, as shown in Figure 2, has been monitored since August 2011. The denitrifying bioreactor consists of two separate compartments with two types of carbon media: woodchips only and woodchips with biochar. The addition of the biochar, a form of organic carbon produced by burning organic material, is a novel media in denitrifying bioreactor research and holds promise for increasing NO3- and P removal. Previous studies have shown that biochar increases microbial activity, which may enhance the rate of denitrification, and reduce nitrogen leaching. Biochar also has the potential to remove P in groundwater by adsorption.**

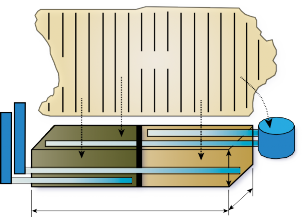
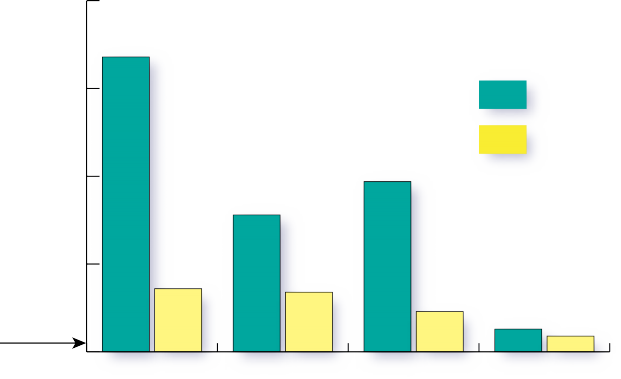
The data displayed in Figure 3 show that the denitrifying bioreactor achieved significant nitrate reductions in groundwater. Nitrate concentrations were, on average 60 percent (and as high as 90 percent) lower in groundwater that had passed through the denitrifying bioreactor than in the groundwater draining from the contributing fields. Groundwater samples were collected from six wells located in the 12 acres of agricultural land draining to the denitrifying bioreactor. The maximum nitrate-N concentration observed in the groundwater is almost 30 ppm, or three times the EPA MCL limit for drinking water, and more than 100 times the levels recommended for stream health. The average nitrate-N concentration observed in the groundwater approaches the 10 ppm drinking water MCL. This specific denitrifying bioreactor includes a runoff collection and dosing system (see Figure 2) to allow treatment of surface runoff in addition to groundwater. The runoff well collected overland flow from the 12 acre contributing area of the farm. Drainage control units (Figure 2) allow for water table control in order to achieve adequate residence time for denitrification to occur as well as for sampling the outflow.
Both the woodchip and biochar beds performed well, and both carbon source materials achieved the same maximum level of nitrate reduction. On average, both substrate treatments in the denitrifying bioreactor were able to reduce nitrate-N to the 0.3 ppm level recommended by the EPA as the maximum concentration to maintain stream health in the Coastal Plain region (Figure 3). These results indicate that denitrifying bioreactor implementation in strategic locations intercepting shallow groundwater and/or run-off has the potential to provide NO3- removal levels on site that translate into measurable downstream water quality improvement.
The denitrifying bioreactor also reduced the dissolved phosphorus concentrations as shown in Figure 4. Note that all phosphorus concentrations observed in the groundwater in this study were higher than the 0.04 ppm level recommended by the EPA as the maximum concentration to maintain stream health in the Coastal Plain region. Although phosphorus does not have direct toxic effects in humans, excess can stimulate the growth of microorganisms undesirable in potable water.
Both denitrifying bioreactors were able to significantly reduce P concentrations in groundwater. The biochar addition substantially increased phosphorus removal as compared to the woodchips alone. The outlet concentration from the biochar treatment approaches the 0.04 ppm maximum recommended level set by the EPA for stream health in this region. Denitrifying bioreactors with biochar amendment have the potential to consistently reduce dissolved phosphorus concentrations by 75 percent or more.
Cost
Denitrifying bioreactors are inexpensive to install and generally maintenance-free. For instance, the denitrifying bioreactors located at the Virginia Tech Eastern Shore AREC cost less than $200 per acre treated. If this is extended out over the expected lifetime of the system (10-15 years), the cost of the denitrifying bioreactor system approaches $10-15 per acre per year. This is comparable to, or less than, other water quality BMPs such as riparian buffers, exclusionary fencing, or nutrient management planning. The only costs are incurred at installation, which include excavation and the purchase of substrates such as woodchips and biochar. If denitrifying bioreactors prove to be a valuable BMP, cost share dollars from federal (such as the U.S. Department of Agriculture-Natural Resources Conservation Services (USDA-NRCS) or state and local (such as Soil and Water Conservation Districts) sources might offset much of the initial cost.
Future Work
Continued study of the Eastern Shore AREC denitrifying bioreactor and other installations in Virginia will focus on monitoring inlet an outlet nitrate-N concentrations in real time in order to develop a nitrogen balance, which will allow for quantification of nitrate removal and assessment of downstream water quality benefits. This work will provide data that can be used to develop denitrifying bioreactor engineering guidelines, inform site selection, and update interim NRCS conservation standards. Additionally, the gaseous products of denitrification, nitric oxide (NO) and nitrous oxide (N2O) and dinitrogen gas (N2), dissolved in the denitrifying bioreactor, will be quantified to ensure that the bio-reactors are not creating an air quality concern. This research will provide insight into the fate of nitrogen in denitrifying bioreactors and allow for estimation of the quantity of reactive nitrogen removed by these systems. Additional data will also isolate the effect of biochar addition on nitrate and phosphorus removal in denitrifying bioreactors.
Acknowledgments
We would like to thank Brain Benham, associate professor at Virginia Tech; Larry Geohring, senior extension associate at Cornell University; Bruce Jones, unit coordinator for Appomattox County Virginia Cooperative Extension (VCE); and Mike Parrish, unit coordinator for Dinwiddie County VCE for their helpful and insightful reviews.
Additional Resources
U.S. Environmental Protection Agency. “Basic Information About Nitrate in Drinking Water.” https://www.epa.gov/system/files/documents/2022-04/ace3-drinking-water-report-section.pdf. (November 2023)
U.S. Environmental Protection Agency. “EcoregionalCriteria.” http://water.epa.gov/scitech/swguidance/standards/criteria/nutrients/ecoregions/index.cfm. (November 2023)
Glossary of Terms
Adsorption – The physical bonding of one substance to another.
Bioavailable—in a form that can be used by organisms (i.e. plants uptake NO3- but cannot use N2.)
Biochar—Similar to charcoal, this form of organic carbon is produced by burning organic material, such as plant material or animal waste, at low temperature in the absence of oxygen. The resulting product is more resistant to decomposition. The method of production determines its best final use, which can be anything from a horticultural soil amendment to the charcoal for a barbeque.
Denitrification—The stepwise transformation of nitrate to nitrite, nitric oxide, nitrous oxide and ultimately dinitrogen gas, which comprises nearly 80 percent of the atmosphere.
Eutrophication—refers to natural or artificial addition of nutrients to water bodies that cause undesired effects, such as algal blooms or lowered dissolved oxygen levels.
Field Scale—Refers to use of a product or methodology in the application for which it was designed as opposed to testing in the laboratory.
Heterotrophic Microbes – Obtain energy, carbon, and reducing equivalents for reactions from organic compounds.
Leach—draining of a dissolved material as it moves with water.
Maximum Contaminant Level (MCL)—the highest concentration of a chemical that can be encountered without adverse affects to human health.
Non-Point Source Pollution—also called diffuse pollution (as opposed to point source pollution that discharges from a defined origin such as a pipe), results from land-use activity and is transported intermittently primarily by rain events.
Reactive Nitrogen—Nitrogen in a form that can participate in chemical or biological reactions, said to be bioavailable, as opposed to nonreactive (inert) nitrogen gas (N2), which is very stable and cannot be used by organisms directly.
* Corresponding author email: zeaston@vt.edu
**Terms defined in the glossary at the end of this publication are italicized the first time they appear in the text.
Virginia Cooperative Extension materials are available for public use, reprint, or citation without further permission, provided the use includes credit to the author and to Virginia Cooperative Extension, Virginia Tech, and Virginia State University.
Virginia Cooperative Extension is a partnership of Virginia Tech, Virginia State University, the U.S. Department of Agriculture, and local governments. Its programs and employment are open to all, regardless of age, color, disability, sex (including pregnancy), gender, gender identity, gender expression, genetic information, ethnicity or national origin, political affiliation, race, religion, sexual orientation, or military status, or any other basis protected by law.
Publication Date
December 8, 2023