Nitrogen Fertilization of Winter Barley: Principles and Recommendations
ID
424-801
EXPERT REVIEWED
Introduction
Modern winter barley cultivars are capable of yields in excess of 170 bu/acre with relatively high test weight. Efficient nitrogen (N) fertilization is crucial for economic barley production and protection of ground and surface waters. Excessive plant-available N produces barley plants that are susceptible to lodging and disease with resulting decreased yields and increased input costs. The potential for enrichment of ground and surface waters with nitrates also increases with excessive N fertilizer applications. However, insufficient N availability to barley plants results in low yields and significantly reduced profits compared to a properly fertilized crop. Nitrogen fertilizer rate and timing are the major tools available after planting to manipulate barley to produce higher yields per acre. The goal of a successful N management program is to match plant-available N with barley crop needs. This paper reports on our current understanding and recommendations with regard to N fertilization of barley.
Barley Growth and N Uptake
Autumn
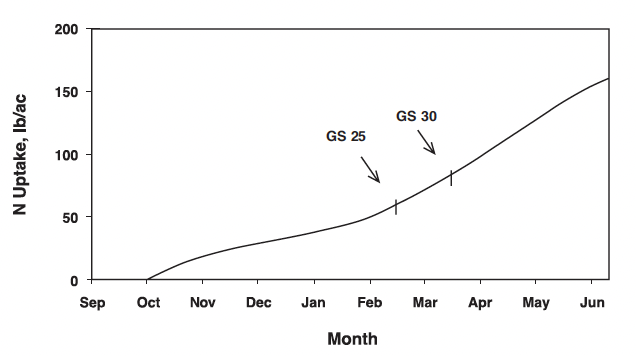
A generalized N uptake curve for winter barley grown in Virginia is shown in Fig. 1. A crop that is planted on time for a particular location germinates, emerges, and tillers prior to the semi-dormant period that generally begins in December in Virginia. Adequate plant-available N at seeding is necessary to promote root growth and moderate tillering of winter barley prior to the semi-dormant winter season in Virginia. Extensive root development during fall growth enhances winter barley resistance to winter-kill and improves early spring growth and nutrient use. Barley planting dates for optimum yields are one to two weeks earlier than for winter wheat, and thus fall N uptake can be greater for winter barley than for winter wheat. Excessive fall barley growth can occur and result in freeze injury to stems and/or early winter lodging of the crop. Such situations have been observed with early planting dates, warm fall temperatures, and excessive plant-available N in the seedbed, particularly from manure or biosolids applications.
Fertilizer N applications of 25-30 lbs N/acre applied in the seedbed at planting are adequate for fall establishment of timely-planted barley. Reductions in this rate should be considered only if the field has a history of manure or biosolids applications, or there is significant residual N from previous fertilizer applications. For example, significant residual soil N might occur following a drought-stressed corn crop. Soil nitrate “quick-tests” can be conducted just prior to planting to determine if high residual soil nitrate levels are present. However, such tests only need to be taken on fields that have received manure or biosolids applications, or have received adequate N fertilization for the summer annual crop such as corn, and yields of the summer crop are low. Contact your local Extension Agent or Nutrient Management Specialist regarding soil nitrate “quick-tests.”
Winter
The barley crop utilizes very little N during the colder temperatures of the winter. Nitrogen applied early in the dormancy period is subject to leaching and/or run-off losses. Applying large amounts of N during January on frozen ground and expecting this N to be available for producing grain in April and May is not reasonable because of our climatic conditions and the growth pattern of winter barley. However, there are situations, particularly on very sandy soils in the Coastal Plain region, in which a small N application in January may be beneficial. If all of the following conditions are met, some N fertilization in January may improve yields. First, significant leaching rains between October and December, for example, >3.5 inches of precipitation during one rainfall event. Second, a thin stand of barley with pale green color due to lack of available N. Third, an expectation for the specific site that several growing days (temperatures of 50°F or greater) will occur in January and early February. Temperatures in January will likely exceed 50°F several days in the Coastal Plain and Southern Piedmont areas, but not in Northern Virginia or the Valley region. Such conditions might warrant an application of no more than 25 lbs N/acre to encourage tillering and root development. However, potential losses to the environment are great with such applications, and they should be made only after careful consideration of the specific field conditions.
Late Winter/Early Spring
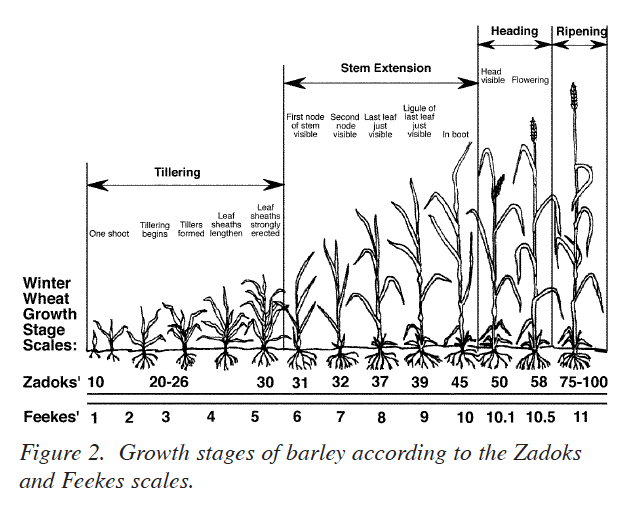
Winter barley generally re-initiates growth in late February or early March with the crop at growth stage (GS) 25 on the Zadoks scale or GS 3 on the Feekes scale (Fig. 2). Growth resumes during this “greenup” period and increased temperatures stimulate additional tiller formation if adequate nutrients, especially N, are available. Since the initial growth is usually rather slow because of cool temperatures, the initial N fertilizer application should be as near to the initiation of growth as it is possible to estimate for the specific site. It is important, however, to realize that fields with low tiller numbers should receive the first N applications so that spring tiller production is not delayed due to a lack of plant-available N. Reference to Fig. 1 shows that N uptake is usually not great (perhaps 30 lbs N/acre) during the period of mid-February to mid-March. Again, this closely matches the growth or dry-matter production pattern for the crop.
Stem Elongation
The leaf sheath erection growth phase, GS 30 (Fig. 2), signals the beginning of stem elongation and the most rapid phase of barley growth. Two important factors are occurring during this time. First, the potential number of kernels per head is being established during the embryonic formation of the head. Second, rapid N uptake begins (Fig. 1).
Management must now be directed to maintaining developing heads. Inadequate available N causes tiller abortion with resulting lowered harvest population. Fields with marginal head populations at the end of tillering are likely to have lower yields due to low numbers of heads/ft2 at harvest.
The initial phase of head development is occurring at GS 30. The late winter/early spring N application should be adequate to develop the embryonic head. Visually, the crop should have a medium to dark green color and be vigorously growing by GS 30. If the crop is beginning to show signs of chlorosis (yellowing), then the application of N at this stage is critical for the development of adequate head size, and for retaining tillers. Priority should be given to N treatments for crops showing a lack of adequate N at any time between green-up (GS 25) and stem-elongation (GS 30).
Research in Virginia with winter barley, during three growing seasons, showed that applying all, or a portion of the spring N application, at GS 30 resulted in economically higher yields at 80% of the sites. Experiments were located in both the Coastal Plain and Valley regions. The research also indicated that the tissue N content of barley at GS 30 can be related to N fertilizer needed to produce optimum economic yields, and that relationship is shown in Fig. 3.
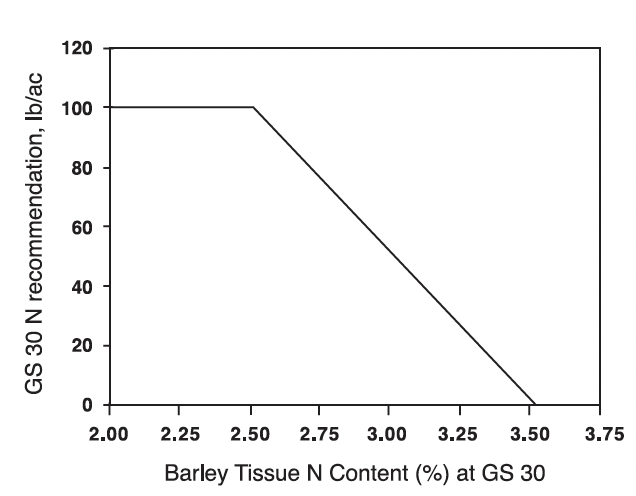
Optimally fertilized barley at GS 30 will contain between 2.7 and 3.5% N in the whole, above-ground plant tissue, which is related to a N fertilizer need of between 70 and 0 lbs N/acre to obtain optimum economic yields (Fig. 3). Barley plants with tissue N contents of 3.5% N or greater at GS 30 do not need additional N fertilization to achieve optimum economic yields. Barley that contains less than 2.7 % N at GS 30 will be pale yellow in color and should have received N fertilizer prior to reaching GS 30. However, total N fertilizer applications at GS 25 and 30 should generally not exceed 100 lbs N/acre due to the lodging potential of barley. Obtaining maximum economic yield responses to N fertilizer applications to barley will often include the use of an anti-lodging growth regulator such as Cerone. Controlling lodging will be especially important where manure, biosolids, or commercial fertilizer N rates exceed 100 lbs of plant-available N per acre applied in the GS 25 plus GS 30 applications.
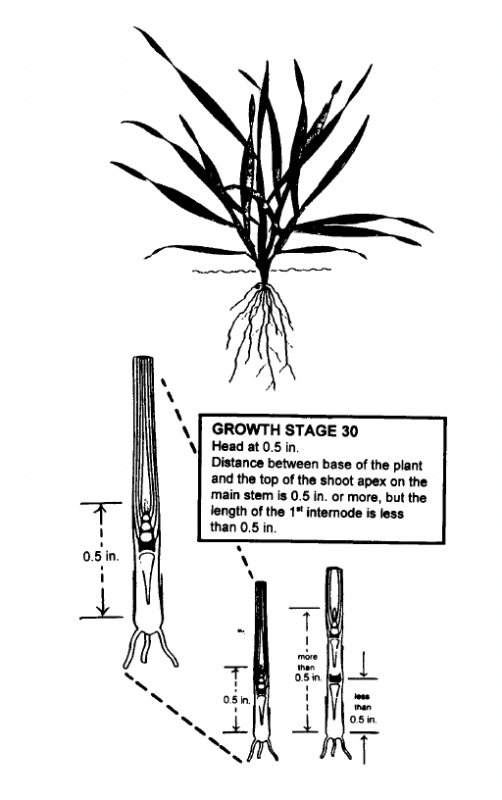
The first requirement for obtaining a good plant tissue sample for use in estimating N fertilizer requirement at GS 30 is to be certain that the barley is in GS 30. Winter barley at GS 30 is illustrated in Fig. 4. Leaf sheaths of the barley are strongly erected and splitting the stem shows a hollow internode area about 1/2 inch in length. GS 31 has been reached when the first node of the stem is visible at the base of the plant (Fig. 2). Sampling at the correct stage of growth is very important. Rapid growth during this time results in the N content being diluted by increases in dry matter production. Samples taken earlier than GS 30 will generally show higher concentrations of N than will be found at GS 30. If these higher %N values were used for predicting the N fertilizer needed on a given field, a less than optimum N fertilizer recommendation would result. Samples taken after GS 30 will usually show lower percent N concentrations which can result in higher than needed N fertilizer recommendations. Thus, proper identification of GS 30 is essential for making good use of this system.
The barley tissue sample is taken by cutting a handful of barley tissue at 20 to 30 representative areas in the field. The top-growth should be cut at approximately 1/2 inch above ground; soil particles clinging to the tissue must be brushed from the tissue; and dead leaf tissue must be removed from the sample. The individual samples should be placed in a paper bag large enough to allow good mixing of the tissue.
After thorough mixing of the tissue sample, take approximately three handfuls of tissue from the mixed sample and place in the sample bag provided by the laboratory, or in a clean paper bag. Samples should go directly to the laboratory. If samples cannot be analyzed within 24 hours from the time they are taken, they must be dried to prevent spoilage. Tissue samples should never be packaged in plastic bags due to condensation that can cause spoilage.
In situations where heavy rains occur during several weeks prior to taking the GS 30 tissue sample, recommendations should be adjusted upward approximately 10-20 lbs N/acre. This is especially true on sandy-textured soils. Situations in which a downward adjustment (10-20 lbs N/acre) of the recommendation should be considered include soils that have received manure or sludge applications, soils with high organic matter levels, and clayey-loamy textured soils. Tissue testing at GS 30 provides an excellent index for growers to use for N fertilizer applications, but values should be adjusted based on experience with individual fields and seasons. Also, slightly higher rates of N fertilizer can be utilized in situations where growers are equipped to make growth regulator applications for lodging control.
Summary
Efficient N fertilization is crucial for profitable barley production and environmental protection. Plant growth and N uptake patterns form the basis for developing an efficient N fertilization program, and N fertilizer applications should be made at times when the plant can utilize the applied N. The N fertilization program must be flexible because optimum N rates vary for soil and season (temperatures and rainfall) conditions.
Adequate N must be present for establishment: Apply 25 to 30 lbs N/acre in the seedbed unless there is a history of manure or biosolids application, or significant amounts of residual soil N are suspected (drought-stressed summer crop).
Growth Stage 25 (late winter/early spring): Apply 0-50 lbs N/acre based on field observations of tiller density and plant color. Fields with tiller densities of greater than 150 tillers/ft2 should receive no N, while fields with densities of <50 tillers/ft2 should receive 50 lbs N/acre as soon as possible.
Growth Stage 30 (stem elongation): Tissue sample and apply N based on tissue N contents, with total spring N applications generally not to exceed 100 lbs N/acre. However, do not wait for GS 30 to take tissue samples if the crop displays visual N deficiency symptoms prior to GS 30.
Acknowledgment
The authors wish to acknowledge the support of the Virginia Small Grains Board and the Maryland Small Grain Growers Association in providing funds to support the research which forms the basis for this publication.
Virginia Cooperative Extension materials are available for public use, reprint, or citation without further permission, provided the use includes credit to the author and to Virginia Cooperative Extension, Virginia Tech, and Virginia State University.
Virginia Cooperative Extension is a partnership of Virginia Tech, Virginia State University, the U.S. Department of Agriculture, and local governments. Its programs and employment are open to all, regardless of age, color, disability, sex (including pregnancy), gender, gender identity, gender expression, genetic information, ethnicity or national origin, political affiliation, race, religion, sexual orientation, or military status, or any other basis protected by law.
Publication Date
July 1, 2024