Virginia Master Naturalist, Basic Training Course, Mammals in Virginia
ID
465-314 (CNRE-176P)
EXPERT REVIEWED


Mammals in Virginia Introduction
Wild mammals provided Native
Americans with food and materials for clothing and shelter. Similarly, colonists relied on native mammals and the domestic forms they brought with them, such as dogs, cats, cattle, sheep, horses, and swine. The beaver, because of the value of its pelt, played an important role in the westward movement of pioneers and the colonization of many portions of North America. Today, domestic and wild mammals play vital roles in the lives of Virginians. Our native mammals are economically, ecologically, and aesthetically important. The study of mammals can be exciting to everyone, and knowledge about mammals should be important to land managers, developers, homeowners, and others whose actions may impact mammals and their habitats.
Mammals are an important topic of study for Virginia Master Naturalists, too. Many mammal species are charismatic and easily capture people’s interest. Mammals also are involved in many wildlife-human conflicts, such as vehicle collisions with deer or bears raiding garbage cans. For these reasons, mammals can make good subject matter for volunteers’ education and outreach programming.
Classification of Mammals
Mammals are members of the phylum Chordata and subphylum Vertebrata, the vertebrates.
The subphylum Vertebrata is comprised of:
- Class Agnatha: jawless forms such as the lamprey
- Class Chondrichthyes: cartilaginous fishes including sharks and rays
- Class Osteichthyes: bony fishes, the most diverse class of vertebrates
- Class Amphibia: including salamanders, frogs, and caecilians
- Class Reptilia: including crocodilians, snakes, lizards, and turtles
- Class Aves: all birds
- Class Mammalia: our primary subject
Table 1 provides a list of all native Virginia mammals, exclusive of truly aquatic forms that may sometimes be spotted in our tidal rivers and along
our shores. The list includes the orders that are represented and the common and scientific
names of each species. Scientific names can be particularly useful when tracking down additional information on a species.
Mammalian Characteristics: What Is Unique About a Mammal?
Mammals share several characteristics with other vertebrates. For example, mammals possess a segmented “back bone” made up of numerous cartilaginous or bony segments known as vertebrae—hence
the name “vertebrate.” Similar to birds, nearly all mammals maintain a constant, internal body temperature despite the outside ambient temperature; they are homeotherms. The heat necessary to maintain body temperature comes from within, through the metabolism of food consumed by the individual— endothermy, not from external sources such as soaking up solar radiation—ectothermy.
Objectives:
- Understand the diversity and distribution of mammals in Virginia
- Describe the role of mammals in Virginia ecosystems
- Describe the natural history and basic biology of mammals
- Describe the adaptations of mammals and how these relate to environmental factors
- Describe the taxonomy of mammals
- Describe the key characteristics used to identify mammals
- Describe threats and/or issues relating to mammals in Virginia
- Understand appropriate techniques and methods for studying mammals
Members of the Class Mammalia display certain unique characteristics that separate them from other vertebrate classes, characteristics said to be diagnostic characters. One of the diagnostic characteristics of mammals is the presence of three bones in the middle ear: the incus (anvil), maleus (hammer), and stapes (stirrup). Birds and reptiles possess only the stapes. Other unique features include the lower jaw being made up of a single bone: the dentary (the left and right dentaries form the mandible); the presence of hair; and milk production for nourishment of young. Hair and milk characteristics will be discussed at greater length later in this chapter.
Diversity and Distribution of Mammals in Virginia
About 100 species of mammals are known to live in or frequent Virginia. Approximately 20 marine mammals—for example, manatees, sea lions, whales, and porpoises—are observed along Virginia’s shores or in its bays and tidal rivers, but many are rare visitors or vagrants from more northerly or southerly areas. The only seal regularly seen off Virginia’s coast is the harbor seal. The manatee, at home much farther to the south, has occasionally been observed during summer in Virginia’s waters over the past 20 years, even sometimes seen in Richmond in the James River. Among the whales, dolphins, and porpoises, the bottle-nosed dolphin is the species observed most frequently on Virginia’s coast; approximately 10 other species have been spotted at least six or more times, including the humpback whale, fin whale, long-finned pilot whale, striped dolphin, harbor porpoise, and the pygmy and dwarf sperm whales. Several other species are known, but largely from occasional strandings along the coast.
Including extirpated and introduced species, 89 species of land mammals have been recorded in Virginia within historic times. Extirpated species include the American bison (Bison bison) and gray wolf (Canis lupus), which disappeared from Virginia in the late 1700s and early 1900s, respectively.
The mountain lion, or cougar (Puma concolor), is another extirpated species. Although sightings have been reported, there are no documented records of a wild form of the mountain lion here or in most of the eastern United States since the early 1900s. The snowshoe hare is on the list; however, that species is likely extirpated; it has not been recorded in many years, despite several attempts to reintroduce it. The wapiti (elk) and the American beaver were successfully reintroduced to Virginia following extirpation in the mid-1800s and early 1900s, respectively. The North American porcupine and the fisher were present in Virginia during colonial times, but were extirpated for more than 120 years. Both species have expanded their ranges into Virginia from West Virginia and western Maryland in the past 15 years or so. The most recently recorded species is the nine-banded armadillo. Several museum specimens and photos of free-ranging individuals have documented its presence in extreme southwestern Virginia since 2019.
Among introduced species are the sika deer that live on Assateague Island. A native of Asia, the sika deer is more closely related to elk than to our white-tailed deer. The nutria—a large, semi-aquatic rodent that is native to South America—occurs in fresh and brackish water marshes in parts of eastern Virginia. This animal was brought to North America
to be raised on farms for what was planned to be a valuable fur industry. When those aspirations did not pan out, many nutria purposefully were released or escaped; as a result, the nutria now inhabits many parts of the southeastern United States, where it outcompetes native wildlife by destroying wetland habitats.
Introduced species that have the greatest economic significance, largely because of their pestilence and the money spent in controlling them, are the house mouse and Norway rat. The house mouse coexists with humans and is a common inhabitant not only of homes, but wherever food is available— especially at warehouses, farms, granaries, and horse- and cattle-feed establishments. The Norway rat is found in similar situations and is well known for the large numbers that can be present and the large quantities of food that may be destroyed. Millions of dollars are spent in attempts to eradicate these species.
Interestingly, there is a good side! Albinistic and other selected forms of the house mouse and Norway rat are bred in captivity and serve as laboratory mice and rats for medical research. Another introduced rat, the black or roof rat, is much less common than the Norway rat, has a longer tail, and, when present in buildings, is typically found on higher floors than is the Norway rat.
The feral hog is another introduced species that is negatively impacting natural habitats in Virginia and is very difficult to eradicate. Escaped groups of domestic hogs take on the characteristics of feral animals within just a few generations, and they pose threats to crops and livestock, as well as native plants and animals.
Eight mammal orders (table 1) are represented in Virginia:
- Didelphimorphia (Marsupialia): opossum
- Cingulata: armadillo
- Soricomorpha: 10 shrews and three moles
- Chiroptera: 17 species of bats
- Lagomorpha: three species of rabbits and the snowshoe hare
- Rodentia: seven squirrels, a beaver, 14 species of mice, voles, and rats, two jumping mice, and a porcupine
- Carnivora: one cat, three canids, bear, raccoon, five members of the weasel family, and two skunks
- Artiodactyla: white-tailed deer, wapiti (elk)
Two aquatic orders represented in Virginia are:
- Sirenia: manatee
- Cetacea: whales, dolphins, and porpoises
Exclusive of bats, about 50 percent of native species have a statewide distribution, about 33 percent are restricted to western mountains of the state, and many of the remaining species occur predominantly in eastern, southern, or southeastern areas. A general characterization of the distribution of mammalian species in Virginia, such as “statewide distribution,” “mountains only,” or “south and
southeastern portions of the state” is provided in table 1. It should be noted that not all species occur at all elevations or in all habitats within a particular region; for example, some species labeled as having “statewide distribution” may occur only at the lower elevations of the mountains.
Similarly, certain species designated as “mountains only” may have very restricted ranges, limited to the highest elevations or small geographic areas within the mountains.
Because of Virginia’s location in the mid-Atlantic region and its broad range of physiographic regions, many northern (boreal) species approach the southern extent of their range in our mountains and, conversely, many southern (austral) species
reach the northern extent of their range in southeastern Virginia. Information about overall distribution of species native to Virginia can be obtained from range maps in field guides or in Internet searches of a species’ range.
Mammal Identification
Some Virginia mammals are well known, and many other species can be identified easily with the help of a field guide. However, certain shrews and rodents are difficult to differentiate— because of subtle differences in morphology, and the skills of a specialist at a museum or academic institution may be required to identify some species. Many of the characteristics described later in the chapter— for example: coloration or amount of bicoloration; body form; length of limbs, tail, and external ears; dentition; and prominent features of the skull— are used to identify individuals to at least the genus level. Because most small mammals are nocturnal or remain hidden from view, there are few opportunities to make measurements and distinguish subtle color patterns.
Rephrasing and adopting an old bird phrase—a mammal in the hand is worth two in the bush—is often an appropriate strategy whenever you want to make positive identifications. For example, when trying to identify unfamiliar mouse-sized or smaller rodent-like animals, one often begins by examining the dentition for the presence or absence of the diastema.
The diastema is the wide space that separates incisors from cheek teeth in rodents, a critical diagnostic characteristic used to separate rodents from other groups. When using field guides, it is also essential to consult distribution maps.
How Do We Learn About Mammals? Traps and Other Devices
If we want to know the kinds of mammals that live on our “back 40,” we probably would not be very successful in documenting those present by relying only on our observations and without using special collection techniques. Some species are difficult to identify, many are active only at night, some are active only in runways hidden under vegetation, some live underground, and still others rarely are seen because they are uncommon, secretive, or timid. In addition to visual observations, which include recording road- killed animals, mammalogists use various kinds of traps and other remote devices.
Live-capture traps, well recognized by both homeowners and professionals, are wire, cage- type traps available in various sizes and used to capture species ranging from chipmunks to foxes and even larger animals. When conducting studies of small mammals, large numbers (up to several hundred) of these “box” traps may be used simultaneously. A popular live- capture trap is the Sherman live trap, an aluminum trap that conveniently folds when not in use to facilitate transport to the field or reduce necessary storage space. Nest boxes for squirrels, box traps for rabbits, and mist nets for bats are examples of other live traps.
Live traps are not always effective for capturing certain species or may be impractical in certain settings; in these cases, kill traps are used. Although the common mouse- and rat- snap traps sold in hardware stores are very familiar to most homeowners, mammalogists use a slightly different model called the Museum Special snap trap. This trap is somewhat larger, more stout (durable), and more effective in capturing small mammals than the hardware-store version of the mouse trap.
A pitfall trap, which is an open- ended container buried upright in the ground so that the top is flush with the surface of the surrounding ground, captures animals that fall or are directed into the open device. Although these devices often are used as kill traps, they can function as a live trap if bedding material and food are placed in the bottom of the trap, and they are checked frequently throughout the time they are in operation. Pitfall traps are very effective for capturing shrews.
Several methods allow detection and identification of mammals without using traps and handling individuals. For example, motion- or infrared-triggered digital cameras can be placed strategically in a variety of habitat types to document the presence and diversity of species in a given area.
Bat detectors that record the echolocation calls of bats are commonly used to determine presence and relative abundance. The recorded calls are compared to a “reference library” of species’ calls from known individuals to identify species.
In a similar fashion, hair can be collected using snares and identified by color, texture, and other features when compared to hairs in a reference library.
Many species, including less- common forms, can be identified on the basis of scats (feces) or tracks. Numerous field guides and Internet resources are available for aid in identification of mammal scats and tracks.
Finally, DNA can be extracted from hair or scat and used to confirm presence of a species and provide details about genetic relationships of individuals.
A Closer Look at Special Mammalian Features
Milk
A diagnostic characteristic of mammals is the production of milk by females for the nourishment of young. The name “mammal” is derived in large part from reference to the mammary gland and mammae—the milk- producing glands and nipples, respectively, from whence young obtain milk. Nipples, the small, outer protrusion of each mammary gland, are typically found in species that bear young that require much parental investment and care early in their development (altricial young).
Mammals of this type often raise their young in nests or other sheltered areas where mother and young are not easily seen and where nursing can proceed uninterrupted. The number of nipples found in a given species is a good indicator of the relative size of the litter (number of young born after a given pregnancy).
Unlike nipples, teats are elongated structures that extend from the area of the mammary glands. Teats are typical of mammals that bear young who developmentally are much farther along and require less parental care (precocial young). Teats are present within members of the Bovidae family, which includes cattle, bison, and the true antelopes; members of the Cervidae (deer) family; and selected other groups. These mammals often live in open areas and are characterized by long limbs and a running type of locomotion. Precocial young are not born in nests or protected areas, they are covered with hair, and their eyes open immediately after birth—in some cases, the eyes may open before they have completely exited the mother.
Importantly, these species are able to walk and follow the mother soon after birth. When feeding, young are able to obtain milk rapidly because milk flows into an enlarged cistern in the teat and the young obtain a relatively large amount of milk with each compression of the teat—just as we would if milking such an animal by hand or with a machine. Because these species represent prey to large predators, the ability to feed rapidly and flee quickly are highly adaptive survival traits.
Composition of Milk: Milk is comprised primarily of water, but it also contains necessary fats, carbohydrates, protein, minerals, and vitamins. Milk composition varies greatly among mammals: rabbit milk is different from fox milk, which is different from deer milk, and so forth. Generally, in species where the young rapidly increase body mass, their milk contains a large amount of fat; in species that display rapid skeletal growth, their milk often contains high levels of protein and certain minerals. The first milk produced by the adult female and initially ingested by the young is known as colostrum.
Colostrum is typically high in fat, other nutrients, and vitamins, and it contains immunoglobulins (antibodies), which are important in preventing infections in newborn mammals. Depending on the species, within hours or days of birth, the immunoglobulins found in colostrum no longer pass through the gut of the young; however, the immunoglobulins obtained earlier from the mother remain in the newborn’s blood until the young develops its own immune system.
Hair
Origin, structure, and types: Hair is another diagnostic feature of mammals. There are two layers to the skin of vertebrates, an outer epidermis and an inner dermis. In reptiles, birds, and mammals, the scales, feathers, and hair are part of the epidermis (i.e., epidermal in origin). In contrast, fish scales are products of the dermis (i.e., dermal in origin).
Along with other functions, the dermis is a support layer for the epidermis. In some mammals, the dermis is thick and especially tough; it is the dermis that we use and prepare to make shoes or leather jackets. A fur coat, though, is comprised of both the dermis and epidermis. The term pelage is used to refer to all of the hair possessed by a mammal, such as when describing “the animal has beautiful pelage.”
In birds, the term for all of the feathers is plumage. Use of the word “fur” usually is confined to describing the soft, dense hair possessed by selected mammals such as mink or muskrat—that is, the fur-bearing mammals.
A strand of hair is an elongated rod (called the shaft) of keratinized (or cornified) cells. Keratin is unique to vertebrates and, in addition to scales, feathers, and hair, keratin is a major component of several other epidermal structures. Hair cells are alive only in the active growth site in the root located at the base of a hair follicle, a tube-like enfolding of the epidermis. It is in the root that this tough, insoluble keratin protein is secreted to form the shaft; as the new growth pushes the hair cells of the shaft outward, the cells die. Pigment (coloration) is added during this initial formative period within the follicle. Hairs that are visible on mammals, including our own hair, contain no living tissue and cannot change color. Hair color sometimes can fade, but color changes only when new hair of a different color replaces the old hair or if the hair is constantly growing and the newer portions display more intense color. As is true in birds when they replace their feathers, mammals molt periodically and old hairs are replaced by new hairs. The new pelage often is the same color as the old, but, in some mammals, juveniles may be characterized by one color, whereas adults are a different color. Hair color also may vary seasonally. A case where hair color differs dramatically between seasons is in the snowshoe hare, where the pelage is brilliant white in winter, but brown in warm months.
The outer layer of cells on a strand of hair constitutes the cuticle—one of three hair layers. These outer cells create a pattern—the cuticular pattern—that often is unique to a certain species. We can use these characteristic patterns, in conjunction with a “key to the hair of local species,” when hair samples found in fecal remains are examined under a microscope as a means to identify the species that was preyed upon. Hair identification thus can help us learn about the diet of a predator, for example a weasel or fox, and also about the kinds of prey species that live in an area where the predator hunts.
Individual strands of hair that comprise wool, the hair produced by sheep, are very fine, often crimped, and the cuticle possesses small barbules that help hold the hairs together. En masse, the hairs create many dead air spaces, which makes wool such a good insulator. It is these barbules that create the prickliness or scratchiness we sometimes feel when wearing wool garments next to our skin.
Sebaceous glands, generally associated with hair follicles but also present in hairless areas, produce an oily substance known as sebum. Sebum helps maintain the health of hair and skin by keeping them from becoming too dry, or conversely—in the case of those species that spend much time in water—protect the hair and skin from being damaged by too much wetness.
Most mammals possess two kinds of hair: very fine under hairs which are short, soft, and dense and comprise the underfur which provides most of the insulation, and the outer guard hairs, which are longer and thicker. Guard hairs contain the pigment that typifies a mammal’s coloration. These sturdier, longer hairs lie over the dense underfur and protect it from abrasion as the mammal moves through its habitat. In nearly all mammals, the hairs are directed posteriorly—toward the tail—so that they do not catch on items as the mammal moves about. Muscles in the skin (arrector pili muscles) cause the hair to stand up or fluff when they contract. By manipulating individual guard hairs, a mammal wears a “light jacket” when the hairs are lying down and directed toward the tail, but it can “slip on a warmer jacket” by activating the muscles to fluff up the fur.
The whiskers on the face of a mammal are called vibrissae. Vibrissae have sensitive receptors at the base of their follicles; when the vibrissae touch something, the receptors are innervated.
Small species and others that are active at night often have especially long vibrissae to help them move among obstacles in their nocturnal activities. Porcupine quills and the hard, sharp spines of some other nonnative mammals are examples of uniquely modified guard hairs. Quills are effective antipredator structures because of small barbules on them that cause them to penetrate only in one direction—deeper into the skin of a predator.
Mammal coloration
Coloration in most mammals is relatively drab compared to other vertebrates, especially the many species of birds with very colorful males. Because many mammals are nocturnal and color cannot be distinguished at night, these species display brown to gray colors that blend well with their night-time haunts. In many cases, the individual hairs on the back and sides are not a solid brown, or gray, or reddish color, but instead have an alternating pattern of light and dark pigments known as agouti. This blended pattern of colors also works well for species that are active night or day, such as voles and cotton rats.
One of the more colorful local mammals is the chipmunk, a diurnal member of the squirrel family. However, the stripes that the chipmunk displays are not intended to enhance its visibility, but just the opposite. These stripes provide nice camouflage in the chipmunk’s natural situations—the coloration blends in with leaves and other features of the forest floor. Most carnivores also display coloration that provides camouflage, presumably so that the prey they seek or larger predators hunting them cannot see them as easily.
The dorsal and ventral surfaces of mammals, such as on the head, body, and tail, often display different colors. This bicoloration is most pronounced in species that are active above groundlevel, for example, mice and other species that climb. Known as countershading, the darker dorsum (what usually would be the light side) and lighter ventral portions (normally the dark side) help break up the animal’s profile by reducing contours and shading—the resulting decrease in contrast confuses the predator.
The mammal skull
No part of a mammal tells us more about that species’ natural history and its relationship to other mammals than its skull. In addition to serving as a head— bilaterally symmetrical animals need a head end—the cranium contains and protects the brain and provides openings for the ears (auditory meatus). Receptors for the critical senses of vision and olfaction, as well as openings associated with gas exchange and nutrition functions, occur within the rostral (face/rostrum) area of the skull.
To facilitate discussion of the features of and differences among mammalian skulls, it would be handy to have a cleaned (skin and flesh removed) rodent skull (e.g., beaver or woodchuck) and skulls from two carnivores with contrasting diets (e.g., raccoon and cat). Why these three skulls? First, bear in mind that mammals have different kinds of teeth (figure 1). Beginning in the center of the jaw, the front-most teeth are the incisors, which are for gnawing or nipping; next are the canines, which often are long and sharp for piercing; and finally are the premolars and molars (the cheek teeth or molariform teeth), which vary greatly in size, number, and appearance depending on the animal’s diet.
The rodent skull is different from the other two forms in that it possesses no canines and displays a wide gap between the incisors and cheek teeth; this space is known as the diastema. Another distinction in rodents is that there are only two upper and two lower incisors instead of four or six, as seen on each jaw in most other mammals. The incisors in rodents grow continuously throughout life and their roots originate well within the skull, away from the exposed portions.
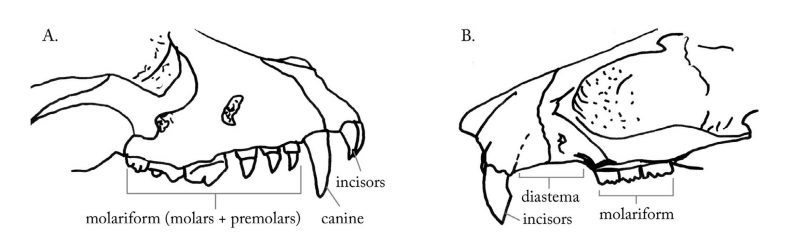
Both the raccoon and the cat skulls possess the full complement of teeth: both have very evident canines, and both have six incisors above and six below (humans have four incisors above and below).
Importantly, dentition adapted for three general diet types are also represented by these three skulls: rodent = herbivore; raccoon = omnivore; and cat = carnivore.
The molariform teeth differ among rodents depending on their specific diet. Those with a diet characterized primarily by grasses typically have cheek teeth with flat, grinding surfaces; those that eat less harsh vegetative matter have cheek teeth with small, rounded cusps.
Comparing the raccoon and cat skulls tells a subtler story. Cheek teeth of the omnivorous raccoon are unspecialized, that is, not very efficient for chewing either harsh vegetation or slicing flesh, but satisfactory for processing many different kinds of food.
Humans are omnivores and have teeth similar to those of the raccoon. Cheek teeth of carnivorous mammals, like the cat, are highly specialized for processing flesh. Although carnivores have fewer cheek teeth than do other mammals, the ones they have display unique piercing and slicing edges. The cheek teeth of canids (e.g., fox, coyote, domestic dog) are somewhat intermediate between those of the raccoon and the cat; piercing and slicing cheek teeth are present, but they also possess teeth for grinding and crushing.
Tooth wear, or lack thereof, is very useful for determining relative age. In studies involving museum specimens, skulls are placed in age classes (e.g., young, young adult, adult, and old adult) based on the amount of tooth wear in the specimens when compared to a known- aged reference specimen. Why is determination of age important? If you are comparing the size of individuals of a certain species from two different locations, you want to be sure you’re not comparing adults from one place to young from the other!
An older adult typically will show much more tooth wear, whereas a young animal will show no tooth wear or the presence of temporary (“milk” or deciduous) teeth. Estimates of age from tooth wear should corroborate estimates from other metrics, such as the use of sutures (joints between bones of the skull). In young, still-growing animals, these “zigzag” sutures are quite evident, and it is easy to differentiate the individual bones that make up the skull. As animals age, these sutures gradually fill in and harden (ossify), making the cranium appear to be made of a single bone rather than the series of individual plates that actually exist.
The various knobs, bumps, and ridges evident on a skull, as well as the bones they closely associate with, tell us much about that animal and play several important roles: They either form part of a joint with another bone, or they serve as a point of muscle attachment. Examples of knobs on the skull that form part of a joint are the occipital condyles. There are many different condyles in mammals, but they all represent “bumps” that form part of a joint. The occipital condyles are located on each side of the foramen magnum (hole in the skull where the spinal cord exits) and articulate with the first vertebra, the atlas. An example of a ridge that serves as a point of muscle attachment is the lambdoidal ridge, located along the upper-back part of the skull of many mammals, where tendons of the major neck muscles attach.
Nails, claws, and hooves
Similar to hair, the nails, claws, and hooves of mammals are epidermal in origin, and keratin is the primary component of these hard structures.
Nails are found on the tops of the digits and are characteristic of species that grasp or hang onto objects (e.g., humans or monkeys hanging onto a branch).
Claws are found at the ends of digits and are curved and sharp at the tips. Claws serve a variety of functions within the species in which they are found. In some instances, claws are used to facilitate digging in the soil or to securely hold onto prey. Claws are also important to species that climb (e.g., tree squirrels), in that these extensions fit into the irregularities of the substrate— tree bark—and may dig in for better grip.
Hooves are weight-bearing structures found at the ends of the digits. The bones of the foot (phalanges, carpals, and tarsals) of the hoofed animals are highly modified and, among certain species, greatly elongated to enhance their running style of locomotion. Among our current native species in Virginia, only the white-tailed deer and the wapiti (elk) possess hooves, whereas many of our common domestic livestock (e.g., horses, pigs, cattle, goats, and sheep) all possess these structures.
Antlers and horns
Antlers and horns are at least partially epidermal and also include keratin. Although antlers often are called horns— especially during deer-hunting season when we hear comments such as, “did you see any horns?” or “he had a big set of horns”—antlers and horns are very different structures. Antlers are characteristic among male members of the family Cervidae—the deer family, which includes elk, moose, and caribou. However, in caribou and domesticated reindeer, antlers are present on females, too.
Antlers are made of solid bone, are shed annually following completion of the rut (the mating season), and often become larger and more elaborate (greater branching) with age. During spring and summer, when new antlers are actively growing, they are enveloped in a layer of skin covered by very fine hair known as velvet—a condition said to be “in velvet.” The skin and velvet give the main beams and tines (points) of growing antlers a very thick appearance. In late summer, when antlers have reached their full growth, the blood supply to the skin is cut off, and the skin gradually dries; the animal works hard to shed or rub off this dead, irritating skin, exposing the solid bone of the antler. Antlers are attached to the skull by bony pedicels on the frontal bones, which are not shed. Discussion of factors affecting antler size in deer is available from numerous sources, including state game agencies. Primary factors controlling antler size are age, genetic factors, and quantity and quality of diet while the antlers are growing.
Horns are possessed by cattle, bison, true antelopes, goats, sheep, and other members of the family Bovidae. Except for special breeds of hornless cattle, horns are present in both sexes; within cattle, one can not tell the difference between a male (bull) and a female (cow) simply on the basis of the presence or absence of horns. Like antlers, horns also originate on the frontal bone. A horn has a bony base that is hollow and covered by a sheath—a highly keratinized structure that antlers lack. It is this sheath, removed from the horns of (dead) cattle, that is often used to create the powder horns used to store black powder for muzzle-loading weapons.
Also unlike antlers, horns are never branched and never shed. The pronghorn, an antelope-like mammal of the western plains of North America, possesses neither true horns nor true antlers, but instead displays a kind of a hybrid structure—the pronghorn. These structures, possessed by both males and females, have a horny sheath that is shed annually, but the small, bone core is not shed.
The mammal external ear
The external ears (pinnae) are the outward features that help us identify mammals and reveal much about a species’ biology. All mammals possess ears—middle and internal ears—but the pinnae or ear flaps (singular pinna) vary greatly among species and can be important in differentiating certain species. But how can pinna size tell us anything about a species’ biology, especially if we have no knowledge about its habitat? Animals that spend much of their time living underground in burrows (said to be fossorial) have very reduced pinnae. The extremes in this case are the moles—fossorial animals that completely lack pinnae. However, most other fossorial species (e.g., weasels, woodchuck [or groundhog]) have very small or reduced pinnae. Shrews and voles also have relatively small external ears; these species spend their time both above and below ground level, within the leaf litter, in runways, or in nests in overgrown fields. However, if somebody handed you a mouse that had relatively large pinnae, such as a deer mouse, it should suggest to you that you are dealing with a species that likely spends considerable time in open areas where hearing is more critical to its survival than if it were underground or hidden by vegetation. Semi- aquatic furbearers represent a second group where the pinnae are characteristically reduced. The beaver, mink, muskrat, and river otter all have very small pinnae. As is true with fossorial species, large pinnae would be disadvantageous to these semi- aquatic animals, because large ears increase drag and hinder movements underwater.
The mammal tail
Obviously, tails are important to the species that possess them. We can also use characteristics about a mammal’s tail as a means to gain information about that species’ biology.
As was true with ear size, tails of a fossorial species and of those that confine their activity to the ground will typically be short. Woodland (pine) and meadow voles are good examples.
Deer mice and many other species of mice and rats have a long tail that serves as a balance and a prop when they climb.
The elongated tail of the opossum is prehensile, meaning it can wrap around and grasp an object. Young opossums sometimes ride on the back of their mother, hanging on to the mother’s tail with their own tails. Opossums also use their tails to carry leaves for nesting, with the tail wrapped around leaves and curled downward and toward their abdomen.
The tail of the beaver is wide and flat and, among other uses, acts like a third leg when the beaver is upright and gnawing on a tree.
Virginia’s two species of jumping mice, the meadow jumping mouse and woodland jumping mouse, have very long tails that act as counter balances. When these mice use their large hind legs and feet to push off and initiate a jump, they don’t want to flip uncontrolled, so the tail provides proper counter-weight to that torque.
The tail of the white-tailed deer is very obvious as it runs away, but when the tail is not erect, the deer blends in with the forest background and makes it more difficult for predators to detect their presence. Does a predator— for example, a cougar or wolf— key in on the tail of their prey?
Perhaps.
Cattle—with a long tail with a brush on the end—possess a great fly swatter.
Not all of the more than 1400 species of bats in the world possess a tail, but all species in Virginia do. All of the bat species in Virginia—but not all bats in the world—have a flap of skin that extends from the tail to the hind legs. This tail membrane— or the uropatagium—serves various roles in flight. In most bats, the uropatagium is naked (i.e., hairless). Interestingly, of the five species in Virginia that roost in the foliage or on trees, the uropatagium is furred. The furred uropatagium is curled under the ventral surface, creating a blanket when the bats are roosting.
Whether short or long, or wide or narrow, naked or hairy, we can learn something about the species’ biology on the basis of its tail.
Mammal Habitats and Special Places
Table 1 presents the relative abundance and general habitat associations of native mammals (exclusive of aquatic species) known to live in Virginia. Many mammal species are considered habitat generalists, which means they can live in a variety of habitat types (e.g., both fields and forests) and survive quite well. Examples of habitat generalists include opossums, short-tailed shrews, woodchucks, white-footed mice, raccoons, and white-tailed deer.
Others species are considered habitat specialists, meaning they are linked closely to a specific habitat type. Examples of species closely allied with forested habitats include smoky shrews, all of the tree squirrels (red, Eastern gray, and Eastern fox), flying squirrels, and gray foxes. Among the old-field habitat species are eastern cottontails, harvest mice, cotton rats, meadow voles, meadow jumping mice, and red foxes. Species associated with a given habitat type sometimes will venture into and take advantage of resources in other habitats, such as squirrels in a field or red foxes in the forest.
Certain species require special conditions above and beyond the dominant types of vegetation that may be present within a particular habitat.
For example, water is critically important to some species and they are never found far from water—whether that be a stream, river, pond, lake, marsh, or swamp. The water shrew, marsh rabbit, muskrat, beaver, river otter, and mink are examples of species in this group. These are semi-aquatic species.
Boreal species, which are distributed more widely north of here—but are found here in Virginia at the southern extent of their range—tend to occur only at higher elevations in the mountains where they find the cooler, moister environments they require. Species such as the rock vole, red-backed vole, northern flying squirrel, and several species of shrews would be affected very severely should these habitats become too warm or dry.
Many species of bats are dependent upon caves and mines, where the ambient temperature is mediated and satisfies their needs for roosting, thermoregulation, and hibernation. Numbers of all cave- dwelling bats in Virginia have dropped dramatically since the arrival of White-nose Syndrome (WNS). WNS is caused by a fungus, Pseudogymnoascus destructans, that first appeared in North America in the winter of 2006-2007. The fungus infects the skin of the muzzles (noses), ears, and wings of hibernating bats. As a result of the infection, individuals experience a cascade of physiologic changes that result in weight loss, dehydration, electrolyte imbalances, and death. Most of the cave bats indicated as “rare” in Table 1 were much more abundant before arrival of WNS.
Roles of Mammals in Virginia’s Ecosystems
Although the role a given mammal species may play in the ecosystem is often expressed through the use of complex ecological models, such models usually are not necessary to observant naturalists who make good use of field observations.
In Virginia, the white-tailed deer is a mammal known to exert considerable influence on forested habitats. Where deer densities are high, their excessive browsing can have profound negative effects on plant abundance, plant-community structure, and the amount of cover or natural shading. Changes caused by deer thus affect the presence and/or abundance of other animals living in that system. Today’s abundance of deer can be attributed to changes in their environment, such as the extirpation of their key predators (e.g., the mountain lion and gray wolf), an increase in the edge habitats in which they thrive, controlled predation by humans in the form of hunting seasons, and more recently, the declining influence of regulated deer hunting.
Predatory species, such as foxes, coyotes, bobcats, and even raccoons, play an important role in regulating the abundance of prey species.
Smaller prey animals, such as voles, characteristically display very high reproductive potentials as a strategy to counterbalance the effects of heavy predation. Because these prolific and abundant species are prime targets of predators, their presence may decrease the amount of pressure that predators exert on other prey species, especially those with lower reproductive potential, thereby helping to sustain a greater overall diversity of species. Such a scenario is most evident in early successional or old-field habitats where a rich mammal fauna exists, consisting of both habitat generalists and old-field specialists.
Despite displaying lower overall abundance than some of these prolific old-field species, certain mammal species—the ecosystem engineers—can still exert considerable influence on habitats through their ability to modify their environments. The beaver is the best known among these engineers in that they build dams, create ponds and wetlands, and alter stream habitats that lie upstreasm of these created features. Following years of accumulation of silt, beaver ponds eventually fill in and may become meadows or even permanent ponds.
The groundhog (or woodchuck) is another mammal well-known for the profound effects it can have on other animals, plants, and soil. Although often a nuisance near buildings and agricultural crops, the woodchuck can also be beneficial. Its abandoned burrows, whether in old fields, forests, or along fence rows, provide shelter for many other mammals of various sizes. In many areas, old woodchuck dens are critical to the survival of rabbit populations; these dens provide rabbits with protective escape sites from predators and suitable climate-controlled shelter during weather extremes.
A final example of the beneficial role mammals can play is provided by moles and their influence on soil ecosystems. The feeding and burrowing activities of moles loosen and aerate the soil, bring leached nutrients up to the surface, enhance drainage, and provide sites for seed germination. Although much smaller than those of the woodchuck, the old burrow systems of moles are often used by voles and shrews.
Conflict with Humans
At the same time that mammals play a key role in Virginia’s natural ecosystems, they also play a major role in human-wildlife conflict. The vast majority of
the calls to Virginia’s Wildlife Conflict Hotline are about mammals, with bears, deer, raccoon, and foxes as the top five species appearing in the call records. Here are some examples of the most-common conflicts: deer, bear, groundhogs, and voles eating crops and garden plants; humans’ garbage, pet food, and bird feeders attracting bears; deer colliding with motor vehicles; and bats, raccoons, mice, and other wild mammals making their homes inside humans’ homes.
Virginia Master Naturalists can have a positive impact by using research-based information to educate the public about the ecology and behavior of mammals and how to coexist with them while reducing negative impacts on both the humans and the wildlife.
Summary
As described in this chapter, Virginia possesses a rich mammal fauna. This richness is due largely to the variety of physiographic regions present in Virginia and the diversity of habitats found in each of those regions. Many different lifestyles are represented among the mammal species that occur here, each of which also demonstrate differences in locomotion, diet, coloration, body form, specializations of the ears and tails, and other features. As you gain additional experience and familiarity with Virginia’s physiographic regions, you should reach a point where you can stand by the side of a stream that flows from a forest into an overgrown field and predict the species of mammals that should be found living in and around these habitats.
Table 1. Native mammals known to occur in Virginia. The list is exclusive of aquatic species. Orders and genera are placed in conventional phylogenetic order; species are arranged alphabetically within each genus. In some instances, distributional ranges may be restricted to highly specialized habitats or small geographic areas. Therefore, it should not be inferred that “MO” indicates that a species occurs throughout all montane portions of Virginia.
Distribution: SW = statewide distribution, MO = mountains only, SE = southern and southeastern portion of Virginia. Abundance: C = common, U = uncommon, R = rare. Habitat: G = generalist, O = old field (overgrown fields, grass/shrubs), F = forested areas.
| Common | Scientific Name | Distribution | Abundance | Habitat |
|---|---|---|---|---|
| Virginia opossum | Didelphis virginiana | SW | C | G |
| Common | Scientific Name |
Distribution | Abundance | Habitat |
|---|---|---|---|---|
| Nine-banded armadillo | Dasypus novemcinctus | Far southwest, expanding northward |
R | G |
| Common | Scientific Name |
Distribution | Abundance | Habitat |
|---|---|---|---|---|
| Masked shrew | Sorex cinereus | MO | C | G |
| Long-tailed shrew |
Sorex dispar | MO | U | Rocky, talus |
| Maryland shrew | Sorex fontinalis | Eastern Shore | R | G |
| Pygmy shrew | Sorex hoyi | SW except Eastern Shore |
U | G |
| Southeastern shrew |
Sorex longirostris | SW except Eastern Shore |
U | O |
| American water shrew |
Sorex palustris | MO | R | Mountain headwater streams |
| Northern short-tailed shrew |
Blarina brevicauda | SW except south central and parts of east |
C | G |
| Southern short-tailed shrew |
Blarina carolinensis | South central and parts of east |
C | G |
| North American least shrew |
Cryptotis parva | SW | U | O |
| Hairy-tailed mole |
Parascalops breweri | MO | U (relatively) |
G |
| Eastern mole | Scalopus aquaticus | SW | C | G |
| Star-nosed mole |
Condylura cristata | SW | U (relatively) |
Near Water |
| Common | Scientific Name | Distribution | Abundance | Habitat |
|---|---|---|---|---|
| Southeastern myotis |
Myotis austroriparius | SE | U | Hollow trees, buildings, and caves where available |
| Gray myotis | Myotis grisescens | MO, southwest |
U | Caves and buildings |
| Eastern small-footed myotis |
Myotis leibii | MO | U | Caves and rocky outcrops |
| Little brown myotis |
Myotis lucifugus | SW | R | Caves and buildings |
| Northern myotis | Myotis septentrionalis | SW | R | Caves and buildings |
| Indiana myotis | Myotis sodalis | MO | R | Caves, hollow trees, under bark, such as on shagbark hickory |
| Eastern red bat | Lasiurus borealis | SW | C | Tree foliage |
| Hoary bat | Lasiurus cinereus | SW | U (sometimes common during migration) |
Tree foliage |
| Northern yellow bat |
Lasiurus intermedius | SE | R | Tree foliage |
| Seminole bat | Lasiurus seminolus | SE | C | Tree foliage |
| Silver-haired bat | Lasionycteris noctivagans |
SW | C | Under tree bark, tree foliage |
| Tri-colored bat | Perimyotis subflavus | SW | R | Caves and buildings |
| Big brown bat | Eptesicus fuscus | SW | C | Caves and buildings |
| Evening bat | Nycticeius humeralis | SW, primarily east |
U | Hollow trees and buildings |
| Rafinesque’s bigeared bat |
Corynorhinus (Plecotus) rafinesquii |
Southwest and southeast |
R | Large hollow trees, cave entrances, buildings |
| Townsend’s big-eared bat |
Corynorhinus (Plecotus) townsendii |
MO | R | Caves near entrances |
| Brazilian free-tailed bat |
Tadarida brasiliensis | SW | U | Caves and buildings |
| Common | Scientific Name | Distribution | Abundance | Habitat |
|---|---|---|---|---|
| Eastern cottontail | Sylvilagus floridanus | SW | C | O |
| Appalachian cottontail | Sylvilagus obscurus | MO | U | Forested brushy areas |
| Marsh rabbit | Sylvilagus palustris | SE | U | Marshy, wet areas |
| Snowshoe hare | Lepus americanus | MO (likely extirpated) |
R | Openings in spruce forests |
| Common | Scientific Name | Distribution | Abundance | Habitat |
|---|---|---|---|---|
| Eastern chipmunk | Tamias striatus | SW except Eastern Shore |
C | G, however primarily near wooded areas |
| Woodchuck (groundhog) |
Marmota monax | SW except extreme southeast and Eastern Shore |
C | G |
| Eastern gray squirrel | Sciurus carolinensis | SW | C | F |
| Eastern fox squirrel | Sciurus niger | mountains and southeast, not between |
U in most areas where it occurs |
F |
| Red squirrel | Tamiasciurus hudsonicus | MO, some areas upper Piedmont |
C | Mixed forests; hardwood forests |
| Northern flying squirrel |
Glaucomys sabrinus | only a few sites in MO |
R | Spruce and fir forests |
| Southern flying squirrel |
Glaucomys volans | SW | C | Primarily hardwood forests |
| American beaver | Castor canadensis | SW | C | Forested areas by streams and reservoirs |
| Marsh rice rat | Oryzomys palustris | middle to eastern |
C | Most often near water, sometimes in dry fields and forests |
| Eastern harvest mouse | Reithrodontomys humulis | SW except Eastern Shore |
U | O |
| Cotton mouse | Peromyscus gossypinus | SE | U | Lowland forests, swamps |
| White-footed mouse | Peromyscus leucopus | SW | C | G |
| Cloudland deer mouse | Peromyscus maniculatus nubiterrae |
MO | C | High-elevation forests |
| Prairie deer mouse | Peromyscus maniculatus bairdii |
Shenandoah Valley |
U | Old-field habitats |
| Golden mouse | Ochrotomys nuttalli | southern half, mountains then to east except Eastern Shore |
U | Disturbed areas in forests, forest edges |
| Hispid cotton rat | Sigmodon hispidus | extreme southwest and southern twothirds, Blue Ridge to east except Eastern Shore |
C | O |
| Allegheny woodrat | Neotoma magister | MO | U | Boulders, cliffs, and caves |
| Southern red-backed vole |
Myodes (Clethrionomys) gapperi |
MO | C | F at high elevation, moss-covered rocks, rotting logs |
| Rock vole | Microtus chrotorrhinus | MO | R | High elevation; cool, moist, rocky areas |
| Meadow vole | Microtus pennsylvanicus | SW | C | O |
| Woodland vole | Microtus pinetorum | SW | C | Brushy areas in forests, edges, sometimes orchards and yards |
| Common muskrat | Ondatra zibethicus | SW | C | Streams near herbaceous growth, marshes |
| Southern bog lemming |
Synaptomys cooperi | mountains and southeast, not between |
U | Bogs, moist meadows, marsh edges, sometimes dry fields |
| Meadow jumping mouse |
Zapus hudsonius | SW | U, sometimes locally abundant |
Usually in fields, sometimes in forests |
| Woodland jumping mouse |
Napaeozapus insignis | MO | U, sometimes locally abundant |
Generally in open areas in forests, often near streams |
| North American porcupine |
Erethizon dorsatum | MO | R | Usually forests |
| Common | Scientific Name | Distribution | Abundance | Habitat |
|---|---|---|---|---|
| Coyote | Canis latrans | SW | U to relatively C in selected areas |
G, but typically near fields |
| Red fox | Vulpes vulpes | SW | C | O and forest edges |
| Gray fox | Urocyon cinereoargenteus |
SW | C | F |
| American black bear | Ursus americanus | SW except Eastern Shore, more abundant in mountains and southeast |
R to relatively C in selected areas |
F primarily |
| Common raccoon | Procyon lotor | SW | C | G, typically most abundant near water |
| Fisher | Pekania pennanti | MO | R | High-elevation forests |
| Long-tailed weasel | Mustela frenata | SW | C | G |
| Least weasel | Mustela nivalis | SW except Eastern Shore, may not be present in southeast |
U | O and forest edges |
| American mink | Neovison (Neogale) vison |
SW | C | Near water |
| North American river otter |
Lontra (Lutra) canadensis |
SW | C east and U in western areas |
Near water |
| Eastern spotted skunk | Spilogale putorius | MO | U | Rocky/boulder areas in forests |
| Striped skunk | Mephitis mephitis | SW | C | G |
| Bobcat | Lynx rufus | SW except Eastern Shore |
U to relatively C in selected areas |
F most often, much cover |
| Common | Scientific Name | Distribution | Abundance | Habitat |
|---|---|---|---|---|
| White-tailed deer | Odocoileus virginianus | SW | C | G |
| Wapiti (elk) | Cervus canadensis | Extreme southwest, reintroduced from western stock |
U, sometimes locally abundant |
G |
Glossary
Altricial – born in an early developmental state, typically requiring much parental care and protection (e.g., opossums, mice, and humans have altricial young)
Colostrum – special milk consumed by young immediately after birth and for a short period thereafter. Antibodies (immunoglobulins) that help protect the newborn mammal are included in colostrum
Condyles – rounded projections on a bone where two bones come into contact, one with the condyle, the other with a depression, to form a joint (e.g., occipital condyles are bumps on the back of the skull where the skull and first vertebra of the “backbone” articulate)
Cuticle – the outer layer of cells on a strand of hair
Dermis – inner layer of skin
Diagnostic character – a characteristic that is unique to a certain group; hair, milk, and three bones in the middle ear are among the diagnostic characters of mammals
Diastema – the wide space or gap between the incisors and the molariform teeth, found especially in rabbits and rodents
Ectothermy – controlling body temperature through external means, such as exposure to sunlight
Endothermy – controlling body temperature internally through oxidative metabolism
Epidermis – outer layer of skin
Extirpated – local extinction, loss of a species from a given area, but it still exists elsewhere
Fossorial – adapted for burrowing and spending much time underground (e.g., moles are fossorial mammals)
Guard hairs – the longer, outer hair that protects the underfur and contains pigment that gives mammals their typical coloration
Homeothermy – maintaining a constant body temperature in a range of ambient temperatures
Keratin – a tough protein that is a major component of hair and nails
Molariform teeth – all the teeth posterior to (behind) the canines (i.e., the premolars and molars)
Pelage – all of the hair on a mammal; the equivalent of “plumage” in birds
Pinnae – external ear flaps, the size of which is a useful indicator of a mammal’s biology
Precocial – born in a later developmental state, such as with eyes fully opened and the ability to walk and follow the mother soon after birth (e.g., deer have precocial young)
Underfur – short, dense, insulating hair located beneath the guard hair
Uropatagium – flap of skin between the tail and the hind legs of bat species
Vibrissae – sensory hairs, including whiskers
Study/Review Questions
- What is a diagnostic character(istic)? List three such characteristics of mammals.
- What is the difference between a precocial (newborn) mammal and one that is altricial? Provide three examples of each type.
- What is the difference between a nipple and a teat?
- Describe general differences in the young and the habitats of animals that possess teats vs. those that possess nipples.
- How can the presence or absence of a diastema be used to differentiate selected mammals?
- List two invasive mammal species currently found in Virginia.
- Describe live traps that might be used for a mouse, a fox, and a squirrel.
- What is meant by the cuticular pattern of hair? What can we learn from that?
- What are arrector pili muscles? Describe two reasons why hair typically lays flat and is directed toward the posterior.
- List—from front to back—the kinds of teeth found in mammals. Which of these are missing in rodents and lagomorphs?
- What can tooth wear and the sutures of the skull tell us about a mammal?
- Contrast nails, claws, and hooves.
- Describe three ways that antlers and horns differ.
- Describe three ways that certain mammals use their tails.
- What is a habitat generalist? A habitat specialist? Name several mammals that fall under each category.
- What is a habitat modifier or a habitat engineer? Provide two examples.
Additional Resources
National Audubon Society. 1996. Field Guide to North American Mammals. New York: Knopf. Reid, F. A. 2006. Peterson Field Guide to Mammals of North America. New York: Houghton Mifflin.
Webster, W. D., J. F. Parnell, and W. C. Biggs. 2003. Mammals of the Carolinas, Virginia, and Maryland. Chapel Hill: University of North Carolina Press.
Linzey, D.W. 2021. The Mammals of Virginia. Second edition. Newark, OH: McDonald Woodward Publishing Company.
Pagels, J. F. and N. D. Moncrief. 2015. Virginia’s land mammals: Past and Present, With Some Thoughts About Their Possible Future. Virginia Journal of Science 66(3), Article 3. DOI: 10.25778/fcfh-4y76.
Virginia Department of Wildlife Resources, Fish and Wildlife Information Database: http://vafwis.org/fwis/.
Virginia Department of Wildlife Resources Help With Human Wildlife Conflicts: https://dwr.virginia.gov/wildlife/nuisance/; toll-free hotline at 1-855-571-9003.
University of Michigan Museum of Zoology, Animal Diversity Web: http://animaldiversity.ummz.umich.
Virginia Cooperative Extension materials are available for public use, reprint, or citation without further permission, provided the use includes credit to the author and to Virginia Cooperative Extension, Virginia Tech, and Virginia State University.
Virginia Cooperative Extension is a partnership of Virginia Tech, Virginia State University, the U.S. Department of Agriculture (USDA), and local governments, and is an equal opportunity employer. For the full non-discrimination statement, please visit ext.vt.edu/accessibility.
Publication Date
February 26, 2024



